Ischaemic heart disease persists as the leading global cause of death and lost life years in adults.1 Angina is a common clinical presentation of ischaemic heart disease related to a supply:demand mismatch of myocardial blood flow, typically provoked by exertion or stress. Invasive coronary angiography is the reference test for angina and identifies obstructive coronary artery disease (CAD) as a cause for symptoms. In Europe and the US, approximately 4 million elective coronary angiograms are performed each year.2,3 However, up to half of all angina patients undergoing elective coronary angiography with symptoms and/or signs of ischaemia have no obstructive epicardial coronary artery disease (INOCA).3 This large, heterogeneous group includes patients with microvascular angina (MVA), vasospastic angina (VSA) or both conditions together. The burden of these conditions on physical and mental wellbeing can be profound; they are associated with morbidity4 and a reduction in quality of life.5 Patients with these conditions commonly attend primary and secondary care, driving up health resource utilisation.6
We propose that optimal clinical management starts with the correct diagnosis; hence we begin by summarising the rationale and protocol for invasive tests of coronary function in INOCA patients. We discuss drivers of myocardial ischaemia and reappraise existing consensus guideline-based management in light of the CORonary MICrovascular Angina (CorMicA) study, the first randomised controlled trial of invasive coronary function testing linked to stratified medical therapy in angina. This review aims to educate and empower the invasive cardiologist to perform vasoreactivity testing and to provide them with an understanding of the positive impact of personalised medicine for individual angina patients. We conclude pointing to future directions in care and the benefits of improved diagnosis linked to translational clinical research to develop targeted disease-modifying therapy.
Background and Aetiology of Angina Without Obstructive Coronary Disease
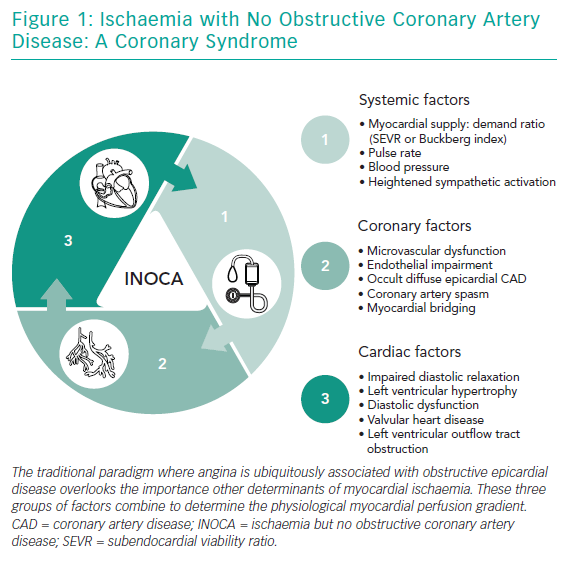
INOCA is a recently proposed ‘umbrella’ term conveying the importance of stable coronary syndromes beyond obstructive CAD (Figure 1). INOCA aligns with the sibling term MINOCA, which stands for myocardial infarction with no obstructive CAD. MINOCA is a similarly diverse syndrome with distinct underlying causes.7
Depending on the patient population studied and the techniques used, between one-third and two-thirds of angina patients with a negative angiogram have an underlying disorder of coronary vascular function.8,9 Importantly, the two most common causes of INOCA (MVA and VSA) are not excluded by a negative non-invasive CT coronary angiogram or invasive coronary angiogram.6 For affected patients, symptom burden, morbidity and health resource utilisation can be considerable.5,10,11
As cardiologists, we often adopt a ‘stenosis-centric’ approach. However, as clinicians we must appreciate the complexity and individual contributors to ischaemia in patients without obstructive epicardial disease (Figure 1). Systemic factors, including heart rate, blood pressure (and their product) and myocardial supply:demand ratio (Buckberg index), are important.12,13 Coronary factors are well recognised, but certain nuances are overlooked. For example, Gould and Johnson recently used their quantitative myocardial perfusion database of over 5,900 patients to show that occult coronary diffuse obstructive coronary disease or flush ostial stenosis may be overlooked on angiography and mislabelled as microvascular angina with suboptimal treatment.14 Other coronary factors that can cause ischaemia and propensity to acute coronary syndromes include structural microvascular dysfunction, endothelial impairment, myocardial bridging and/or epicardial vasospasm.15,16
The final group of factors that can drive INOCA is cardiac, including left ventricular hypertrophy or restrictive cardiomyopathy where subendocardial ischaemia results from challenges with arteriolar vessels penetrating deeper into the myocardial tissue with shorter diastole and enhanced systolic myocardial vessel constriction.17 Heart failure (with reduced or preserved ejection fraction) can lead to elevated left-ventricular end diastolic pressures that reduce the physiological myocardial perfusion gradient.
Valvular heart disease, e.g. aortic stenosis or left ventricular outflow tract obstruction, is a well-recognised cause of INOCA, although controversy exists over whether symptoms in mechanical outflow tract obstruction (aortic stenosis) relate to microvascular dysfunction, supply:demand factors or both.18 Most experts support haemodynamic factors as the main cause of ischaemia here, especially since symptoms and coronary flow reserve improve immediately after valve replacement.19
Non-invasive Functional Testing
Non-invasive tests provide indirect assessments of myocardial resistance by assessing perfusion during exercise or pharmacological stress, typically using systemic adenosine. Nevertheless, perfusion assessment lacks the sensitivity to diagnose the relative contributions of epicardial and microvascular disease to myocardial blood flow reduction. In addition, some patients with a propensity to vasospastic chest pain syndromes may have normal findings from pharmacological and exercise stress testing. This review focuses on the invasive diagnosis and related management of angina subjects without obstructive disease; the non-invasive workup is covered elsewhere.20,21
Diagnosis and Rationale for Invasive Testing
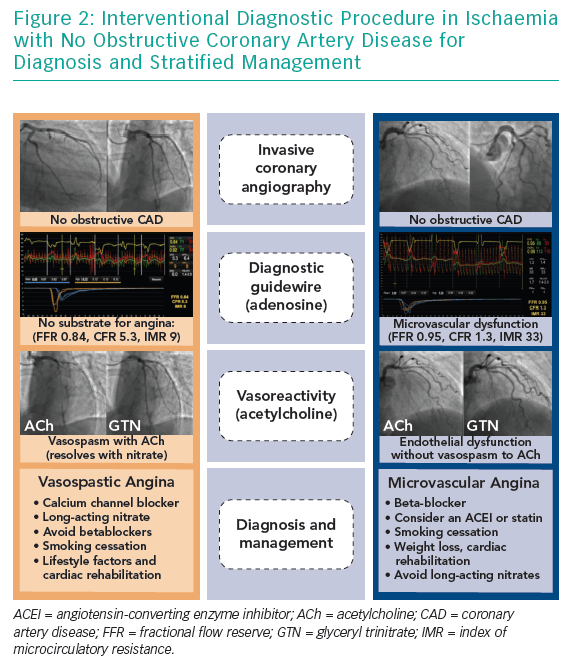
In the cardiac catheterisation laboratory, coronary vascular function may be assessed ad hoc during the patient’s index coronary angiogram. This often involves an interventional diagnostic procedure (IDP) where a guidewire-based assessment of coronary blood flow is performed at rest and during interrogation with pharmacological probes, typically adenosine and acetylcholine.
The rationale for an IDP is three-fold. First, these patients often present with typical angina for invasive coronary angiography, which offers an opportunity for the cardiologist to provide patients with an accurate diagnosis and explanation for their symptoms. Second, discrimination of MVA, VSA and non-cardiac chest pain permits distinct treatment outlined in consensus practice guidelines. Third, evidence of coronary vascular dysfunction carries prognostic insights for patients and their clinicians. However, in contemporary standard practice, additional invasive tests on patients with unobstructed coronary arteries are very rarely performed.
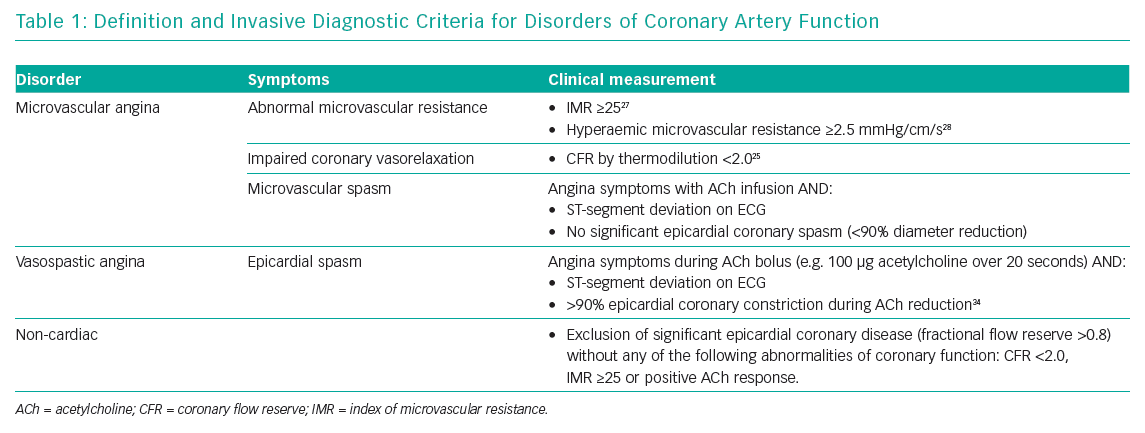
The IDP consists of two steps: assessment of coronary circulation vasorelaxation using invasive coronary physiology at rest and with hyperaemia; and second, assessment of the propensity of the coronary circulation to excessive vasoconstriction using intra-arterial acetylcholine (microvascular and/or epicardial vasospasm) (Table 1). We typically prefer the left anterior descending coronary artery as the target vessel because it subtends the largest myocardial mass. While regional microvascular dysfunction is well recognised, interrogation of multiple vessels increases the procedural duration such that the benefits of testing may be outweighed by the risks.
Assessment of Coronary Vasorelaxation and Resistance Using Diagnostic Guidewire
The purpose of step one is to assess the coronary flow reserve (CFR) and microvascular resistance, typically using the index of microcirculatory resistance (IMR; Figure 2). Flow-limiting epicardial coronary disease may be assessed using fractional flow reserve (FFR), which is the ratio of mean distal coronary pressure to mean aortic pressure at maximal hyperaemia. Abnormal FFR is defined as ≤0.80 or alternatively a non-hyperaemic pressure ratio with different cut-off may be used, e.g. diastolic-only pressure ratio.22,23 The CFR is determined by dividing the hyperaemic coronary blood flow by the resting flow. This is also termed the vasodilator capacity and reflects the ability of the coronary circulation to augment blood flow from rest. CFR is calculated using thermodilution as the resting mean transit time divided by hyperaemic mean transit time; an abnormal CFR is defined as ≤2.24,25 Microcirculatory resistance can be assessed using thermodilution or Doppler. The IMR is calculated as the distal coronary pressure at maximal hyperaemia multiplied by the hyperaemia mean transit time.26 Increased IMR (≥25) is representative of microvascular dysfunction.27 If Doppler wires are used, the hyperaemic microvascular resistance may be calculated as the ratio between hyperaemia distal coronary pressure and hyperaemia average peak velocity, with measurements >2.5 mmHg/cm/s being abnormal.28
In brief, 50–70 U/kg intravenous heparin should be administered and a guiding catheter used to engage the coronary artery. We induce hyperaemia pharmacologically with intravenous adenosine 140 μg/kg/minute, although other pharmacological agents or exercise may be used. A pressure–temperature sensor guidewire (PressureWire X™, Abbott Vascular) or a Doppler wire (ComboWire XT® or FloWire®, Philips Volcano Corporation) may be used. In this technique, the guidewire wirelessly transmits data to a workstation or computer using dedicated analysis software (e.g. CoroFlow™, Coroventis). Typically, intra-arterial glyceryl trinitrate is given as for standard FFR assessment, although we suggest using ≤200 μg. The half-life of glyceryl trinitrate is around 2 minutes; thus after 10 minutes only 3% of the medication is active and it is unlikely to suppress a false-positive test for vasospasm in step two. Conversely if acetylcholine (ACh) testing is performed first, then resting flow and CFR assessment may be inaccurate, particularly after a positive vasospasm test.
After equalisation and passing the diagnostic guidewire into the distal third of the vessel, the blood flow at rest is assessed either by thermodilution (akin to right heart catheterisation with Swan–Ganz/bolus of normal saline) or by Doppler wire.
Assessment for Propensity to Coronary Vasoconstriction: Acetylcholine Provocation
In healthy endothelium, ACh stimulates abluminal release of nitric oxide, mediating vascular smooth muscle relaxation and increased blood flow. At high doses or in patients with endothelial dysfunction, ACh directly stimulates vascular smooth muscle, causing vasoconstriction that can precipitate epicardial vasospasm and/or microvascular vasospasm-induced ischaemia. Typically, infusions of ACh at concentrations approximating 0.182, 1.82, and 18.2 µg/ml (10−6, 10−5 and 10−4 mol/l, respectively) at 1 ml/min for 2–3 minutes are given via a mechanical pump. These doses were historically derived using experiments adopting subselective infusion through an infusion catheter into the left anterior descending artery, assuming a resting flow of 80 ml/min. The effective concentration at tissue level was estimated at 10−8 to 10−6 M. The assessment of Doppler response to ACh involves intracoronary infusion catheters in combination with Doppler wire and requires larger guiding catheter sizes (7 Fr) and a 3 Fr infusion catheter into the coronary artery.
Centres in Japan with over four decades of experience with ACh testing adopt a pragmatic and streamlined approach using sequential bolus doses of ACh via the guiding catheter. Doses start from 20 µg, increasing to 50 and 100 up to 200 µg in the left system (or 20, 50 and 80 µg into the right coronary) over 20 seconds followed by up to 3 minutes between doses.29 Coronary angiography is performed when either ST segment changes or chest pain (or both) occur, or after 1 minute following the completion of each injection. We routinely use a well-engaged guiding catheter to deliver ACh via a 2-minute infusion using an external mechanical pump without an additional infusion catheter. This approach facilitates smaller guiding catheters and reduces risk, time and procedural cost.
Epicardial coronary artery spasm is defined according to the Coronary Vasomotion Disorders International Study Group criteria whereby chest pain is reproduced with ST segment deviation and ≥90% vasoconstriction to 100 µg of ACh (5.5 ml of 10−4 M over 20 seconds). Microvascular spasm is defined chest pain and ST segment deviation without significant luminal constriction (<90%) and represents a functional subtype of microvascular angina. Severe epicardial endothelial dysfunction is defined by ≥20% luminal constriction during ACh infusion (up to 10−4 M); this finding implies a significant reduction in coronary artery blood flow with prognostic implications when compared with patients whose arteries are <20% constricted.30 Bradycardia with ACh is common and usually self-limiting, although a reduced dose during interrogation of the right coronary artery (maximum 50–80 μg ACh) may reduce occurrence.
CorMicA and Clinical Evidence
Despite a wealth of clinical evidence from observational studies, until recently there has not been a single randomised controlled trial of coronary function testing. In the absence of randomised trials demonstrating patient benefits, observational evidence has rarely been applied in practice. CorMicA now provides proof-of-concept clinical evidence to support the case for patient benefits when management is guided by invasive tests of coronary artery function (IDP). Ad hoc adoption of coronary function testing for patients with INOCA is currently restricted to a few interested academic centres. In part this relates to lack of evidence that an IDP has clinical utility or improves patient well-being.
Supported by the British Heart Foundation and the patients who kindly agreed to take part, we delivered the CorMicA trial to specifically address this gap in evidence.31,32 We hypothesised that stratified medicine, including an IDP with linked medical therapy, would be routinely feasible and lead to improvements in angina and quality of life in patients with no obstructive CAD.
A total of 391 patients with definite or probable angina, as determined on the Rose angina questionnaire,were enrolled over a 12-month period from November 2016 at two large tertiary referral centres serving around half the population of Scotland (approximately 2.5 million people).33 Coronary angiography revealed no obstructive CAD in 185 (47%) of the patients who completed the Rose questionnaire and 151 individuals were immediately randomised to one of two arms: the intervention group (stratified medical therapy, IDP disclosed) or the control group (standard care, IDP sham procedure, results not disclosed). The mean age of subjects was 62 years and 74% were female.
The diagnostic intervention included a guidewire-based assessment of a major coronary artery, usually the left anterior descending coronary artery, followed by pharmacological coronary reactivity testing (Figure 2). This diagnostic assessment aligned with contemporary guidelines.34,35 The IDP involved measurement of CFR (abnormal <2.0), microcirculatory resistance (IMR; abnormal ≥25) and FFR (abnormal ≤0.80). Vasoreactivity testing was then performed by infusing incremental concentrations of ACh followed by a bolus of ACh of up to 100 μg to assess for epicardial or microvascular vasospasm. The diagnosis of a clinical endotype (MVA, VSA, both or none) was linked to distinct guideline-based management stratified by diagnosis.36 The primary endpoint was the mean difference in angina severity at 6 months as assessed by the Seattle Angina Questionnaire summary score.37
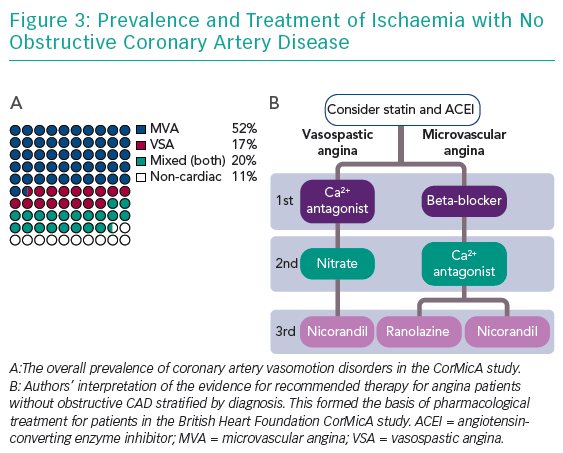
In an all-comers study design, the IDP revealed isolated MVA in 78 subjects (52%), isolated VSA in 25 subjects (17%), mixed angina (both) in 31 subjects (20%) and non-cardiac chest pain in 17 subjects (11%) (Figure 3). The intervention was associated with a mean improvement of 11.7 units in Seattle Angina Questionnaire summary score at 6 months (95% CI [5.0–18.4], p=0.001). In addition, the intervention led to improvements in the mean quality of life score (EQ-5D index 0.10 units; 95% CI [0.01–0.18]; p=0.024) and visual analogue score (14.5 units; 95% CI [7.8–21.3]; p<0.001).31 Notably, after the disclosure of coronary function testing, over half of the treating clinicians changed their diagnosis. There were no differences in major adverse cardiac events after 6 months of follow-up (2.6% controls versus 2.6% intervention; p=1.00). Thus, we showed that in patients undergoing invasive coronary angiography, obstructive coronary disease is excluded in half of all patients; and within this large group of patients, the majority have a readily identifiable disorder of coronary vasomotion. Specifically, the IDP with linked medical therapy was routinely feasible and safe, resulting in improvements in angina and quality of life at 6 months in this group of patients. CorMicA was undertaken in a real-world setting and the results appear to be transferable to clinical practice. Future trials are anticipated to determine the wider external validity of this approach.
Stratified Medicine in Angina
We start by considering the patient in the context of non-coronary contributors to INOCA (Figure 1). Non-pharmacological therapies encompassing lifestyle modification, risk factor control, evidence-based pharmacological therapy and patient education are also essential for stratifying treatment. Lifestyle recommendations are covered in detail in the recent European Society of Cardiology guidelines.38 We will focus on the two most common diagnostic groups to guide distinct medical treatments.
Microvascular Angina
The diagnosis of MVA may be suspected in angina patients without obstructive CAD who have evidence of microvascular dysfunction. In the IDP above, microvascular dysfunction consists of abnormal CFR (<2.0), abnormal IMR (≥25) and/or microvascular spasm during ACh provocation. Clearly, this is a heterogenous entity akin to the syndrome of heart failure with preserved ejection fraction (HFpEF) with diverse aetiology.39
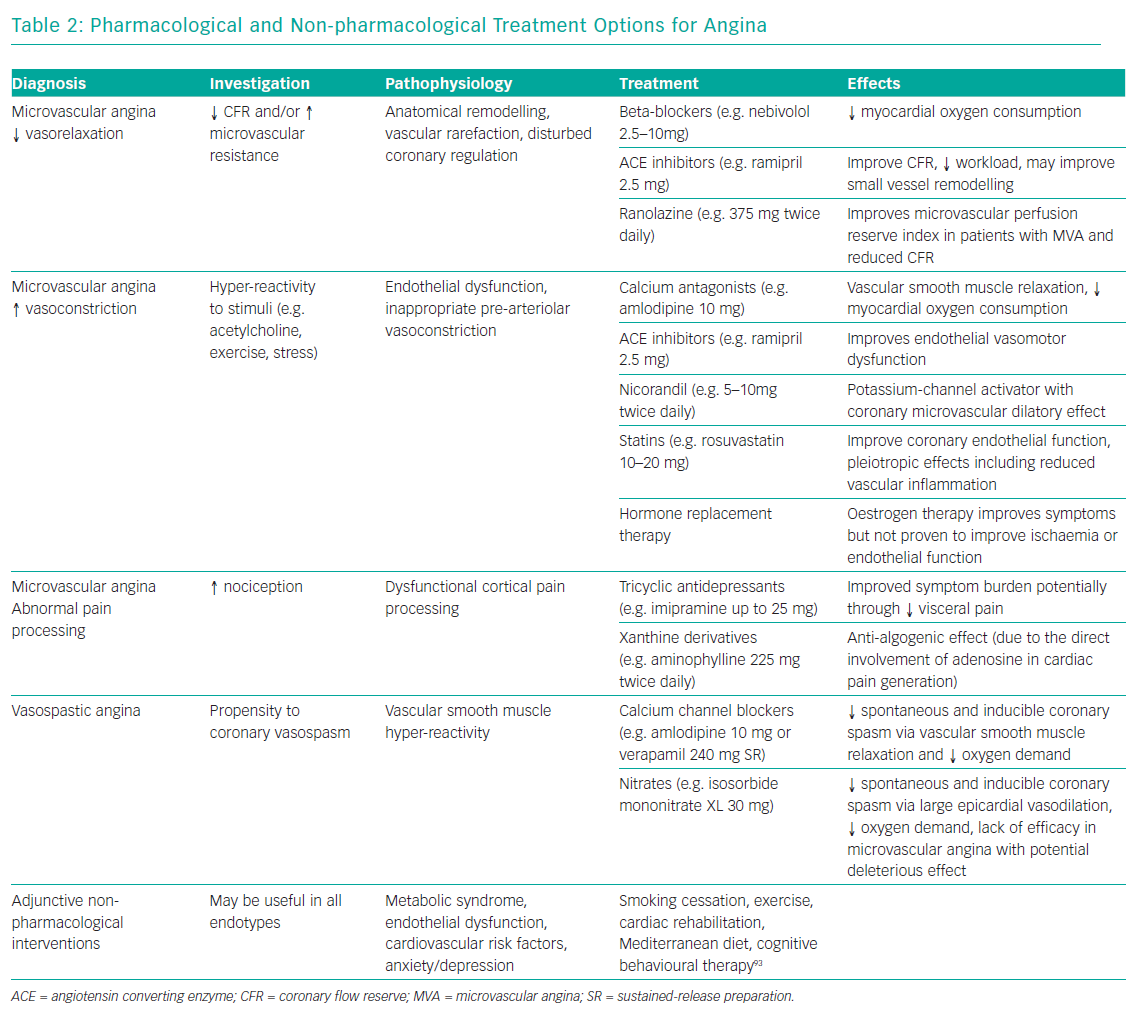
Baseline “disease-modifying” therapies that have demonstrated benefit in clinical trials of microvascular angina include angiotensin-converting enzyme inhibitors40 and statins.41 We particularly support these baseline therapies in patients with diffuse CAD without epicardial obstruction. European Society of Cardiology guidelines for patients with MVA recommend beta-blockers as first-line and calcium antagonists if the former are not tolerated or efficacious (Table 2).36 Dihydropyridine calcium blockers, e.g. amlodipine 5–10 mg, may be added to beta-blockers if blood pressure permits. There is accumulating evidence that long-acting nitrates are ineffective or even detrimental in MVA.42 Lack of efficacy may relate to poor tolerability, steal syndromes through regions of adequately perfused myocardium and/or related to the reduced responsiveness of nitrates within the coronary microcirculation.43 There is significant clinical overlap between MVA and HFpEF,44 so inferences about nitrate response may be drawn from the Nitrate’s Effect on Activity, Tolerance in HFpEF (NEAT-HFpEF) study. In this randomised controlled trial, HFpEF patients on isosorbide mononitrate actually did worse with reduced activity levels assessed using an accelerometer.45
Ranolazine is a relatively new and well-researched antianginal therapy that may improve myocardial perfusion by decreasing sodium and calcium overload, thereby improving myocyte relaxation and ventricular compliance.46 In a randomised placebo-controlled clinical trial of ranolazine led by the Women’s Ischemic Syndrome Evaluation investigators, although there were no overall improvements in angina and myocardial perfusion with ranolazine, patients with a reduced CFR (<2.5) benefitted from ranolazine, with significant improvements in myocardial perfusion (p=0.014) and angina frequency (p=0.027).47 Multiple other drugs that reduce angina may be added, including nicorandil and ivabradine.21
Vasospastic Angina
VSA is often characterised by rest angina, often with preserved effort tolerance. The poor nitrate response or tolerance seen in MVA contrasts with patients with vasospastic angina, in whom nitrates are a cornerstone therapy and beta-blockers are relatively contraindicated.36 Dual pathology (VSA with underlying microvascular disease) is not uncommon.48,49
A positive diagnosis of VSA facilitates treatment using non-dihydropyridine calcium antagonists, e.g. controlled-release diltiazem at up to 500 mg daily, which are usually very effective. High doses of calcium channel blockers (non-dihydropyridine and dihydropyridine) may be required either alone or in combination. Overall, calcium channel blockers are effective in treating >90% of patients.50 Unfortunately, ankle swelling, constipation and other side-effects may render some patients intolerant. Long-term nitrates may be used with good efficacy in this group.51
In about 10% of cases, coronary artery spasm may be refractory to optimal vasodilator therapy and require large doses of calcium-channel blockers or nitrates. Alpha-blockers, e.g. clonidine, may be helpful in selected patients with persistent vasospasm. In patients with poor nitrate tolerance, the potassium-channel-opener nicorandil can be tried. In refractory cases of VSA in patients with acute coronary syndrome, coronary angioplasty may be a useful bailout option.52 In such recalcitrant disease, it is worth reappraising the underlying diagnosis and considering coronary vasculitis as a presentation of multisystem disease.53
Beyond Pharmacotherapy
The importance of addressing lifestyle factors cannot be overemphasised, particularly given that half of the patients in the CorMicA study were clinically obese. Strategies to help address this, including exercise programmes and cardiac rehabilitation, may help facilitate important long-term lifestyle changes.51 Additionally, a new diagnosis of angina may increase the use of non-pharmacological therapies, including cardiac rehabilitation which may benefit patients with ischaemic heart disease.54,55 After clarifying the diagnosis, patients may be more motivated to pursue important lifestyle changes, including diet, exercise and smoking cessation. We are assessing these and longer-term events according to randomised group at 12 months.
In the CorMicA study, we noted significantly lower illness perception scores at 6 months among the intervention arm, representing a less threatening view of illness. Angina reduction and improved quality of life scores could be in part related to better patient understanding and a less threatening perception of the illness. Longitudinal studies of other cardiovascular diseases have shown that illness perception is an important predictor of longer-term outcomes, including disability and returning to work.56 The Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina (ORBITA) trial highlights a placebo effect and we support that the positive diagnosis may be therapeutic in itself.57 Angina symptoms are often subjective and multifactorial in origin, so patient education and validation of symptoms may facilitate further improvement.
Future Directions
Our hope is for a personalised medicine approach whereby patients with different angina subtypes, defined by the results of coronary function tests, may benefit from targeted therapy. Further research is needed to determine whether this approach may lead to patient benefits. More widespread invasive testing allows identification of diagnostic subgroups for the development of targeted therapies guided by mechanistic studies. We recently identified systemic vascular abnormalities in patients with MVA and VSA, highlighting a potential therapeutic role for endothelin-receptor antagonists targeting the ETa receptor.58 In addition, Rho-kinase inhibitors represent a potential future therapeutic option with anti-effects in patients with excessive vascular smooth muscle constriction. More research is needed in well-defined patients endotypes (subgroups).
Conclusion
Patients with INOCA present a diagnostic and therapeutic challenge to physicians. MVA and/or VSA are the two most common causes of INOCA and may be overlooked using anatomical coronary tests alone. Invasive diagnostic testing permits a positive diagnosis to be made, or excluded, during the patients’ index presentation. Correct diagnosis of the underlying cause of angina permits stratified treatment of the distinct disorders (MVA, VSA or non-cardiac chest pain). CorMicA has shown this approach to be safe, feasible with demonstrable benefit for patients.