The treatment of coronary bifurcation lesions (BL) is still challenging for interventional cardiologists, due to the relatively high-risk of the side branch (SB) closure and the increased long-term restenosis.1 The idea of a dedicated bifurcation stent (DBS) was proposed as a solution for problems associated with the BL treatment by means of a classical stent.1 Three groups of stents are available at the moment: a proximal main vessel (MV) stent (Axxess™, Devax, US), MV stenting across the SB with different designs making possible permanent access to the SB, and finally, purely SB dedicated stents (Tryton™, Sideguard™ and Biguard™). Neither of these stents match proximal–distal MV size difference nor take into account vessel angulations. The Bifurcation Optimisation Stent System (BiOSS®) (Balton®, Poland) is completely different from the above systems.
Device Description
BiOSS Expert (Balton, Poland) is a coronary bifurcation balloon-expandable stent made of 316 litre stainless steel with strut thickness of 120 micrometre (μm) and covered with a mixture of a biodegradable polymer and paclitaxel – an antiproliferative drug. The coating process of the stent by a biodegradable polymer and paclitaxel uses the same technology as the Luc-Chopin2 stent developed by Balton. The polymer layers release paclitaxel in a time-controlled process of their slow biodegradation (lasting eight weeks), inhibiting a neointima formation process.2
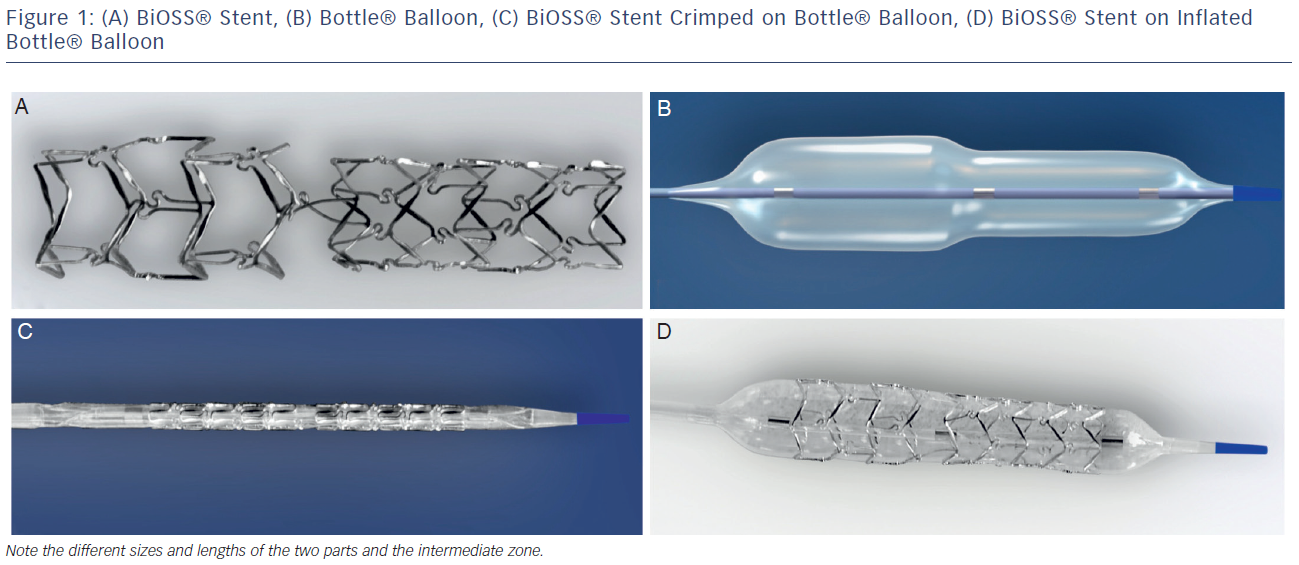
The stent consists of two parts, proximal and distal, connected with two connection struts (depending on size 0.9–1.5 mm, average 1.2 mm long) at the step-up middle zone (see Figure 1A). The proximal part of the stent has a larger diameter in relation to the distal part. The diameter ratio of proximal to distal parts varies between 1.15 and 1.3, ensuring physiological compatibility and optimal flow conditions. There are three lengths (15, 18 and 23 mm) of BiOSS stents available on the market. The proximal part is always a bit shorter than the distal one (average 1 mm). The nominal foreshortening of the stent is less than 0.5 %. The stent strut/vessel area ratio varies between 15 and 18 %.
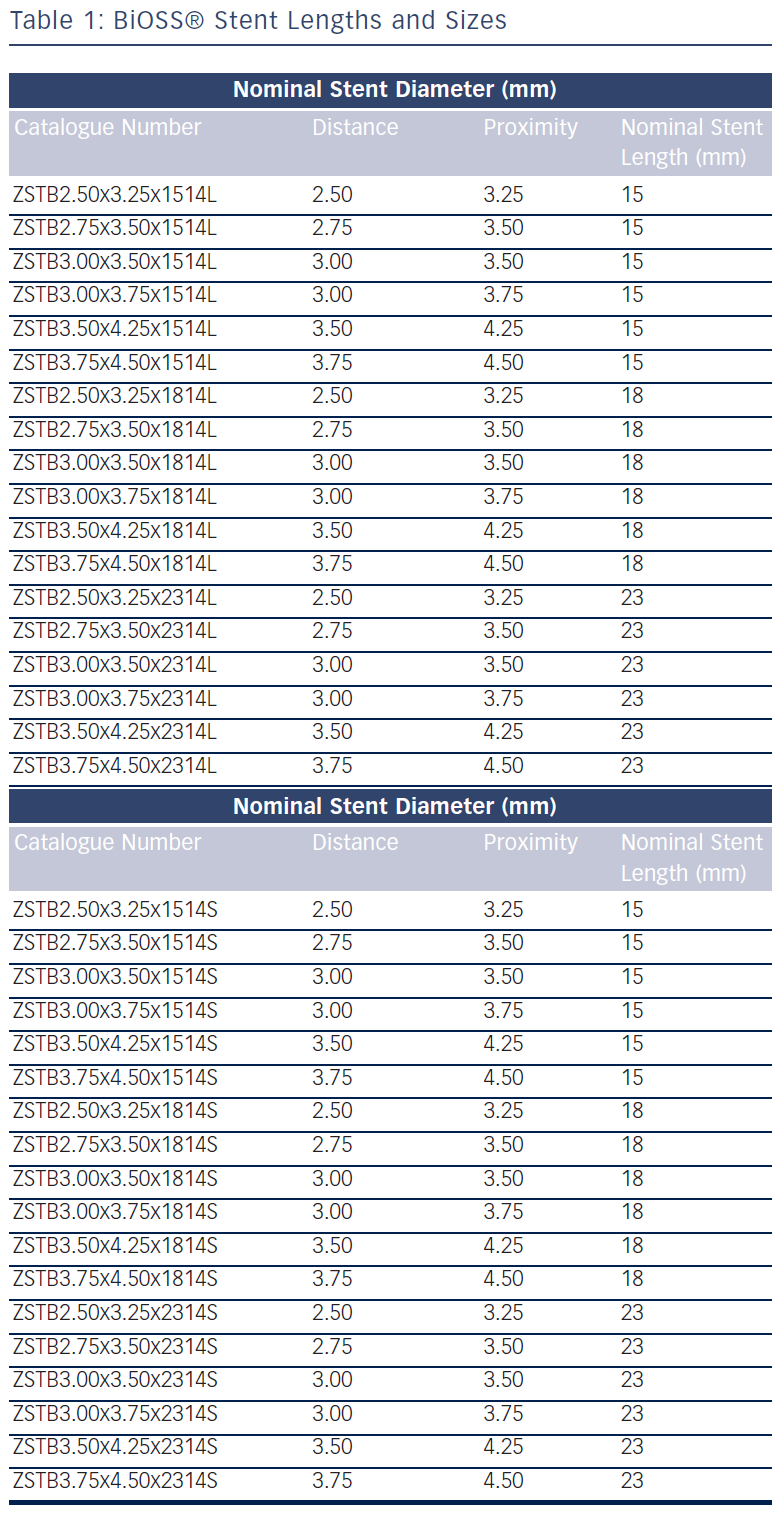
The stent is crimped on a bottle-shaped balloon (Bottle®, Balton, Poland). Bottle balloons are available in a wide range of sizes and lengths allowing the left main stem treatment as well (see Table 1). The balloon nominal pressure is 10 atmospheres (atm), whereas the rated burst pressure is 18 atm. The balloon is semi-complaint with an increase in a diameter size of 0.25 mm at 14 atm, both proximally and distally (see Figure 1B).
The delivery system for the BiOSS stent is a rapid exchange system compatible with 0.014-inch guidewires and with 5 French (1.63 mm internal diameter) guiding catheters. The BiOSS stent is introduced over a single guidewire, which (opposite to many other dedicated systems guided on two guidewires) eliminates the risk of wire wrap (twisting) or other complications with double guidewire driven systems (see Figures 1C and D).
BiOSS – the Mechanism of Action
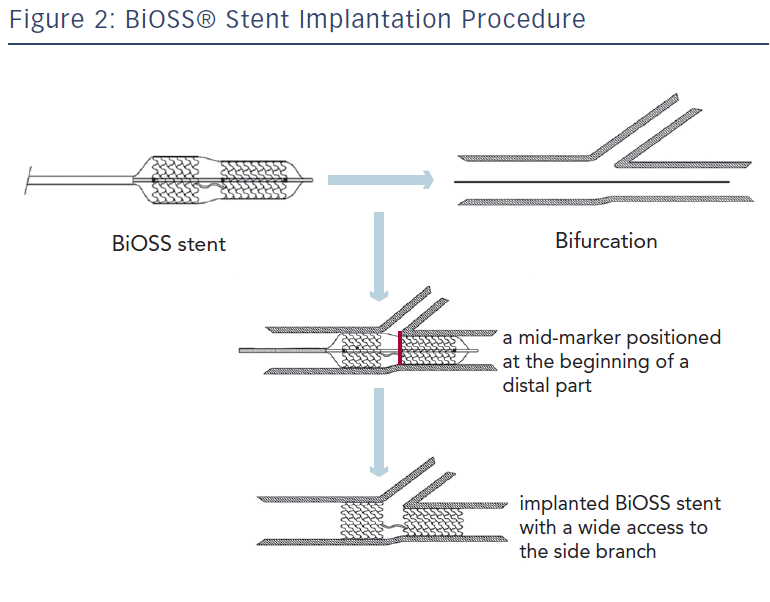
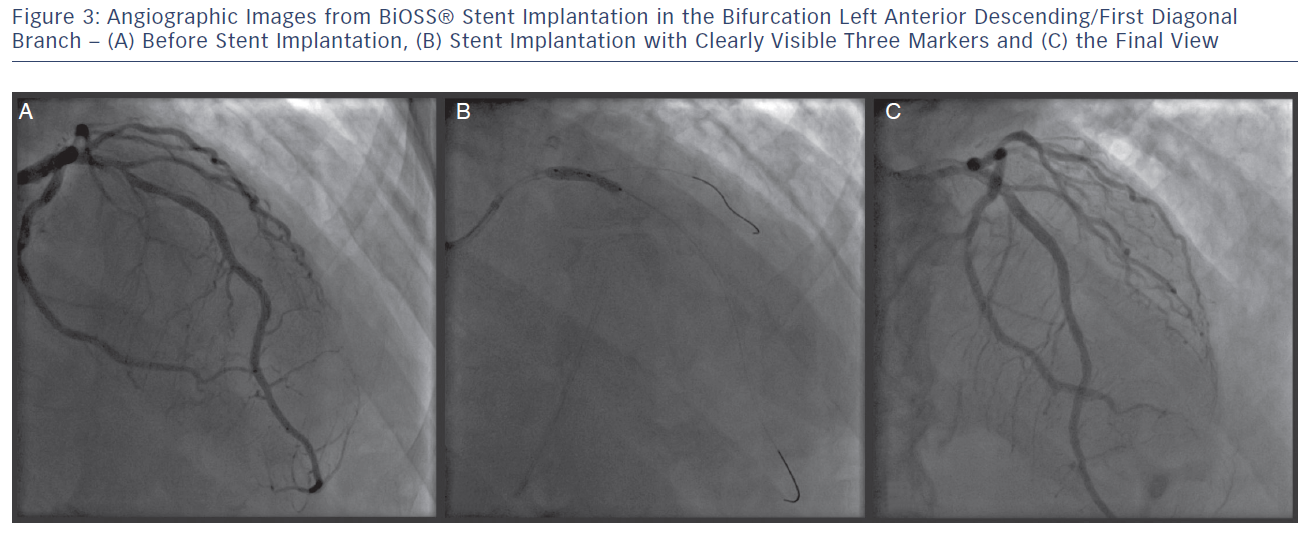
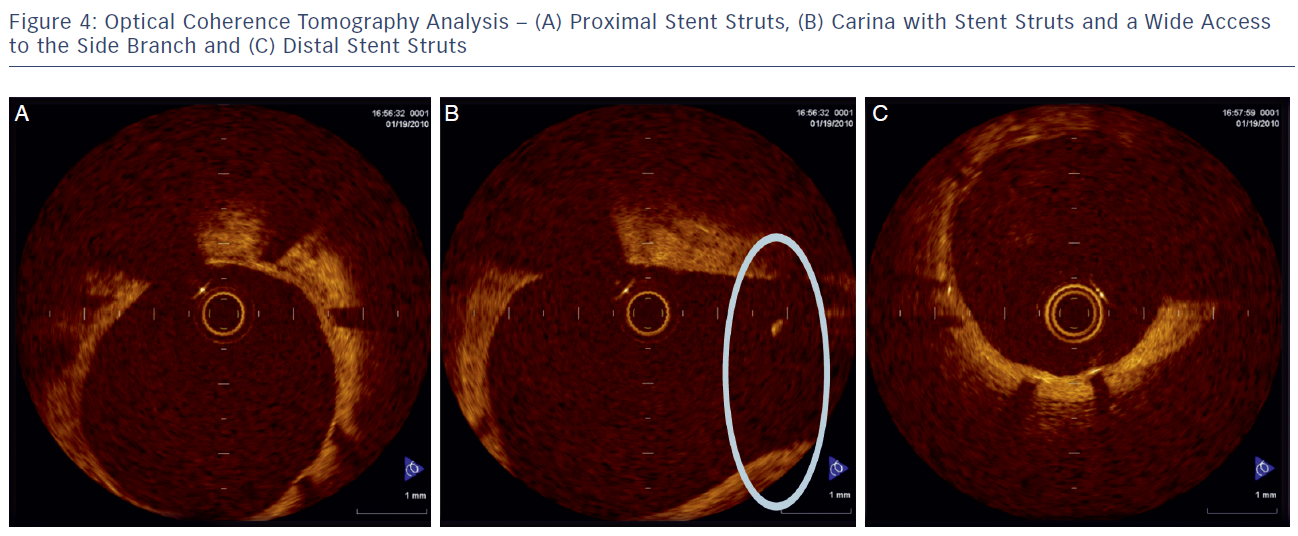
The delivery balloon for the BiOSS stent has three markers (proximal, middle and distal), which assures the exact stent placement at the point of the bifurcation. The proximal end of a mid-marker is again positioned exactly at the beginning of a narrower distal part for the correct implantation. The 1.2 mm intermittent zone ensures ‘self-positioning’ of a stent after balloon deflation as well as wide opening to the SB. After BiOSS stent implantation (during which the main vessel segment is straightened), the proximal and distal main vessel segments return to their initial position/angulation, opening the side of the stent to the branch. The wider proximal part of the stent ensures lateral stretching of the SB lateral wall counterbalancing the carina displacement from the distal part of a stent (see Figure 2). Moreover, this stent provokes much less carina displacement because it mimics the exact bifurcation construction with the proximal – distal main vessel diameter mismatch. These changes result in the proximal and distal parts adequate configuration, matching vessel exact sizes. The specific configuration of the stent system ensures a ‘kissing-like’ effect. Figure 3 depicts angiographic results of BiOSS Expert stent implantation, whereas Figure 4 presents intravascular ultrasound (IVUS) and optical coherence tomography (OCT) analysis, respectively.
From the beginning, BiOSS stent construction has questioned whether a 1.2 mm long intermediate zone of a stent was the weakest part, predisposing to restenosis and intrastent thrombosis. Recently published results from a three-month study3 and the known results of a 12-month study4 of BiOSS Expert Registry deny those assumptions. In addition, our IVUS study showed that BiOSS stent provides a comparable increase of the lumen in most stenosed parent vessels (MV and MV) to a classical stent. Simultaneously, BiOSS stent construction (of course in case of proper implantation) provides better access to the SB in comparison with the classic drug-eluting stents (DES). It was proven by the significantly bigger window length found in the BiOSS group – a parameter, which represents the access to the SB.5 The analysis of the plaque, lumen and vessel areas show that BiOSS stent construction assures the realisation of the proximal optimisation technique (POT), which is strongly recommended by the European Bifurcation Club.1 In addition, smaller variability in vessel and lumen areas at the level of the distal limb after BiOSS stent implantation was found. This was clearly connected with carina and plaque shift after stent implantation. These findings confirm that BiOSS stent construction limits carina and plaque shift towards SB, which are two major factors responsible for the SB compromise.5
Clinical Applicability
BiOSS Expert stent should be classified as DBS treating MV and ensuring the SB access. Our experience showed that this device is very user-friendly. The vast majority of implantations were possible using radial access (>90 %) and 6 French compatible equipment (including also left main [LM] cases). Moreover, such a stent ensured ideal immediate efficacy (100 % device success rate). It is worthwhile stressing how easy rewiring of the SB is after BiOSS Expert stent implantation. This device’s success rate is clearly superior to all other reported procedural success rates of dedicated coronary bifurcation stents (100 % for BiOSS Expert versus 75–95 % for other types of devices). This high success rate is a result of the obvious lack of problems frequently occurring with other dedicated devices, like guidewires criss-crossing, improper device orientation and big device profile.4
Moreover, in our studies an additional stent in SB was necessary in only 10 % of patients (majority of them had a procedure on the left main stem [LMS]), which confirms that the BiOSS stent fits well to the provisional T-stenting (PTS) strategy. A good angiographic result was achieved with a low rate of final kissing balloon technique (only in 13 % of cases), which is sometimes very demanding, especially for inexperienced operators. It suggests that a bottle-like shape of a stent delivery balloon and the balloon used for post-dilatation (Bottle) is associated with a kissing-like result.4
The analysis of 12-months worth of angiograms revealed a rather small (p<0.01) reduction of minimum lumen diameter (MLD) for the MV and the main branch (MB) (12.1 % and 14.0 %, respectively) and a significant increase of MLD for the SB (19 %). In association with such a process, the percentage diameter stenosis (DS) increased both in the MV and MB, while it decreased in the SB. The late lumen loss was significantly different for the MV and MB (0.46 mm and 0.39 mm, respectively) and very small (<0.1 mm) for the SB. It is not easy to explain this phenomenon; however, it seems rational to believe that optimised flow conditions due to the BiOSS design with less effect of shear stress are responsible.4 It is very likely that the ongoing randomised trial, which aims to compare BiOSS Expert stent and classical DES (POLish Bifurcation Optimal treatment Strategy [POLBOS study]) will show real clinical usefulness of this novel device.
BiOSS Lim – Sirolimus Version
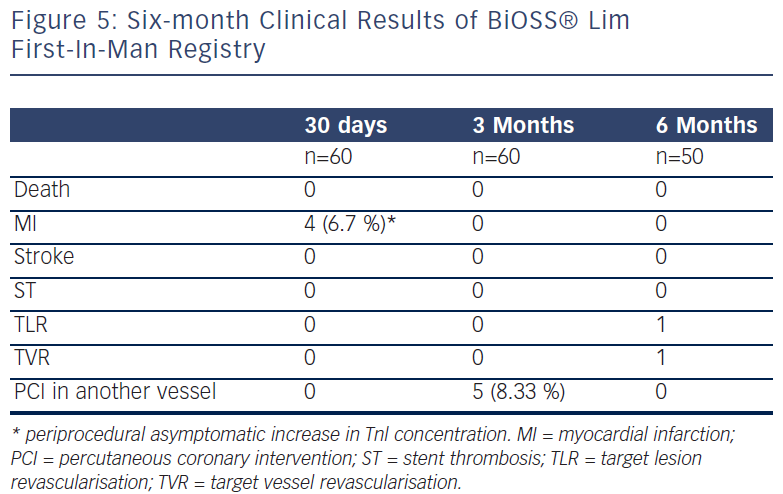
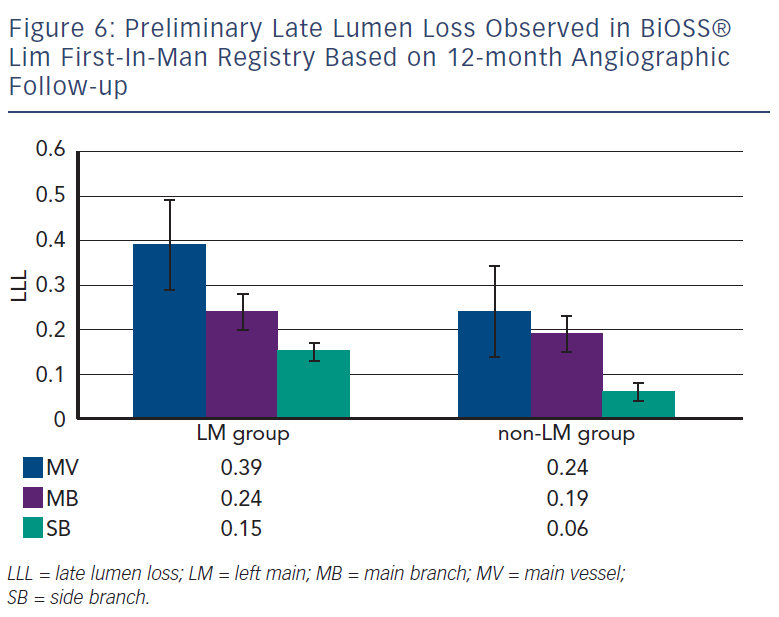
Recently, Balton has started a First-In-Man (FIM) study with a sirolimus version of the BiOSS stent (BiOSS Lim). Very promising results for a study performed in normal nonatherosclerotic porcine coronary arteries (both inflammation and injury scores were very low, and relatively small neointimal proliferation) showed that the BiOSS platform with sirolimus is more potent than paclitaxel and would ensure excellent clinical results. Recently, data collected from the BiOSS Lim FIM study prolonged this belief (see Figure 5). We proved that the late lumen loss after BiOSS Lim implantation is smaller when compared with the paclitaxel-eluting version BiOSS Expert (see Figure 6).
Conclusions
Our experience with the BiOSS stent led us to believe that this device is easy to use and compatible with modern low size equipment, shortening the procedural and fluoroscopy time and decreasing the contrast volume.4,6 The elimination of carina displacement (as a main mechanism of SB compromise) by BiOSS stent keeps the side branches patent and does not require further treatment. In diseased vessels, with atherosclerotic plaque presence, these could be translated into a smaller number of peri-procedural myocardial infarctions and less late stent thromboses.