Cardiogenic Shock, One of the Unresolved Problems in Cardiology
Provided the patient with acute myocardial infarction (AMI) reaches the hospital, he has a more than 90 % probability to survive.1 However, when cardiogenic shock develops, either initially or in the course of the infarction, only one in two patients is alive one year later.2,3 It really seems that all the progress in the treatment of myocardial infarction (MI) within the last ten years has bypassed these 5–10 % of patients suffering from cardiogenic shock complicating myocardial infarction – the publication of the most important evidence-based progress in treatment of cardiogenic shock after acute myocardial (CSAMI) patients – the earliest possible reperfusion of the culprit lesion of the occluded infarct coronary artery by percutaneous coronary intervention (PCI) or aortocoronary bypass (ACB) – is already 14 years old.4 This disappointing situation explains the urgent search for better treatment concepts5 to lower this unacceptably high mortality of patients with cardiogenic shock complicating myocardial infarction.
About 80 % of cardiogenic shocks after AMI are due to left ventricular pump failure; the rest consists of severe mitral regurgitation due to papillary muscle dysfunction or rupture, ventricular septal rupture, cardiac rupture, shock due to right ventricular infarction and other rare causes.6 In this article we will mainly focus on cardiogenic shock due to left ventricular failure after AMI.
Which Patient with Cardiogenic Shock After Acute Myocardial Infarction Will Survive?
Cardiogenic shock is not only a problem of the heart. One main cause of the high mortality among patients with CSAMI is the development of prolonged shock leading to systemic inflammatory response syndrome (SIRS) and even sepsis,3,7–9 with consecutive development of deleterious multiple organ dysfunction syndrome (MODS).10,11 Consequently, CSAMI is not just a disease of the heart, but a disease of the critically ill intensive care unit (ICU) patient with SIRS and MODS. This has to be taken in mind when trying to improve prognosis and reduce mortality by simply increasing cardiac output and stabilising blood pressure.
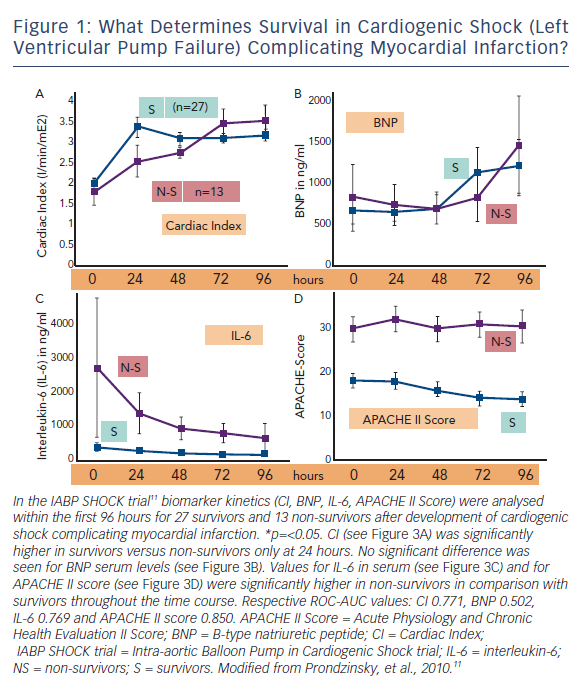
Figure 1 shows the time course of cardiac index, brain-type natriuretic peptide (BNP), interleukin-6 (IL-6) and Acute Physiology and Chronic Health Evaluation II (APACHE II) score in surviving and non-surviving patients with cardiogenic shock due to left ventricular failure complicating MI.
Of course one would expect that haemodynamic measures like cardiac index would discriminate best between survivors and non-survivors. And indeed, cardiac index in survivors was significantly higher than in non-survivors, but only less than 1 L x min-1 x m-2 and only within the first 24 hours (see Figure 1A). The heart failure marker BNP was even without any prognostic relevance within the first 96 hours (see Figure 1B). On the other hand, IL-6 serum levels as a marker of SIRS were much higher in non-survivors (see Figure 1C) as was also the severity of disease score APACHE II, a measure of MODS (see Figure 1D). Quantifying these findings and extending the results to other trials12 one can clearly demonstrate that MODS – as measured by the APACHE II score or the Simplified Acute Physiology Score (SAPS II) score – is a stronger predictor of death than all haemodynamic measures; and even SIRS parameters like IL-6 are as predictive as classical haemodynamic measures like cardiac output (see Figure 1).
The consequence of these findings is that our therapeutic attempts must not only achieve successful reperfusion of the occluded coronary artery and improvement of cardiovascular function, but also optimal intensive care for prophylaxis and treatment of SIRS and MODS, especially within the first hours and days. These aspects are often neglected, but will determine the outcome of the patients with cardiogenic shock.
What Do the Guidelines Tell Us?
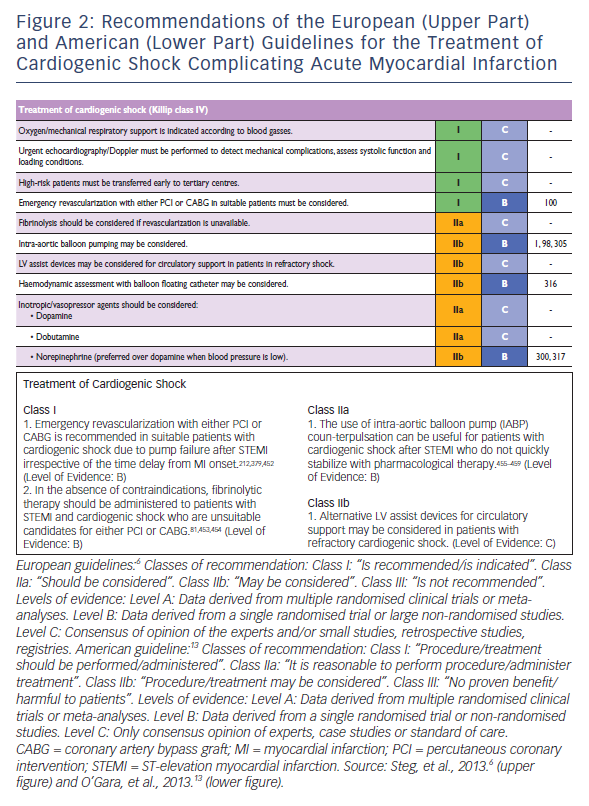
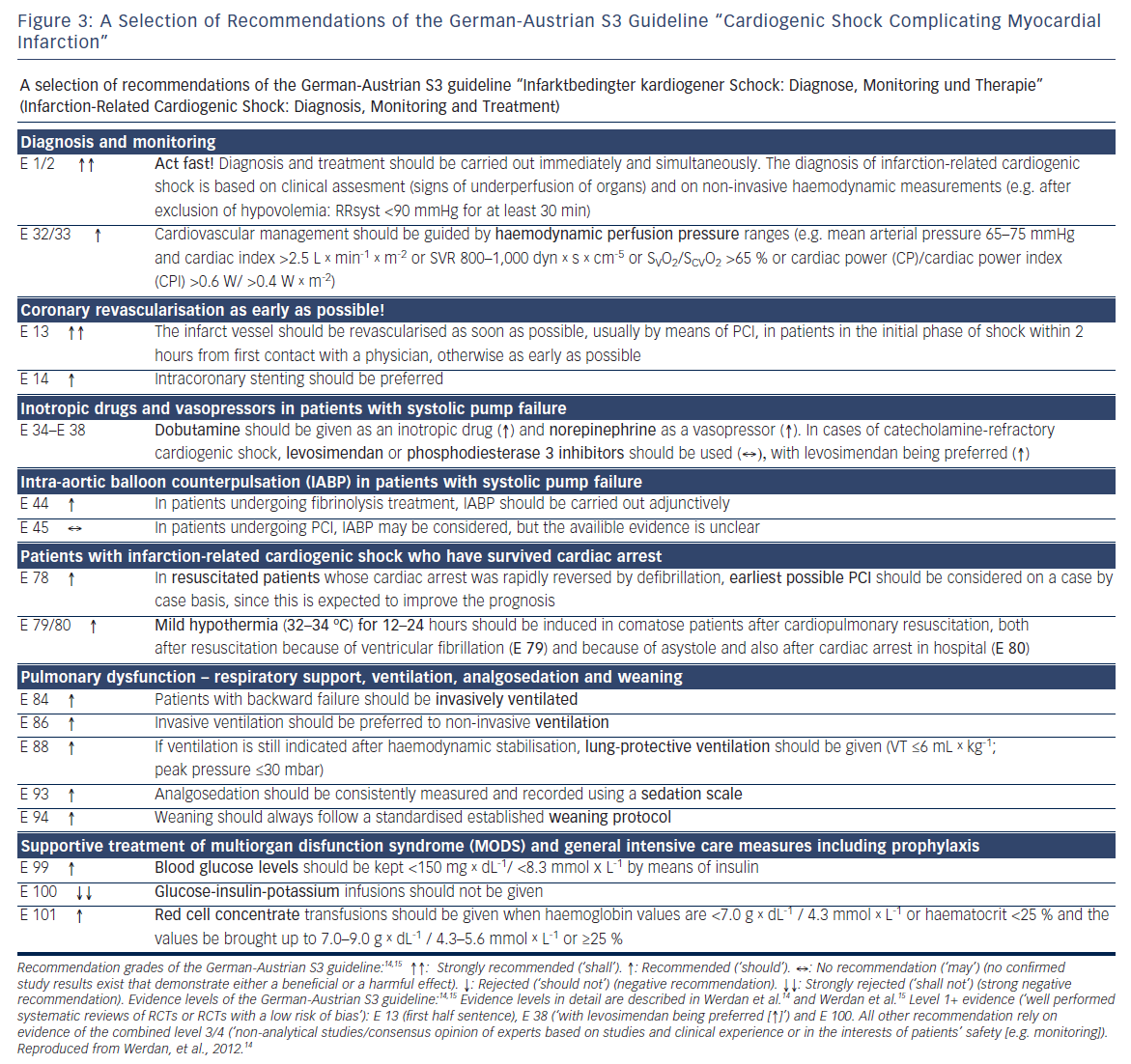
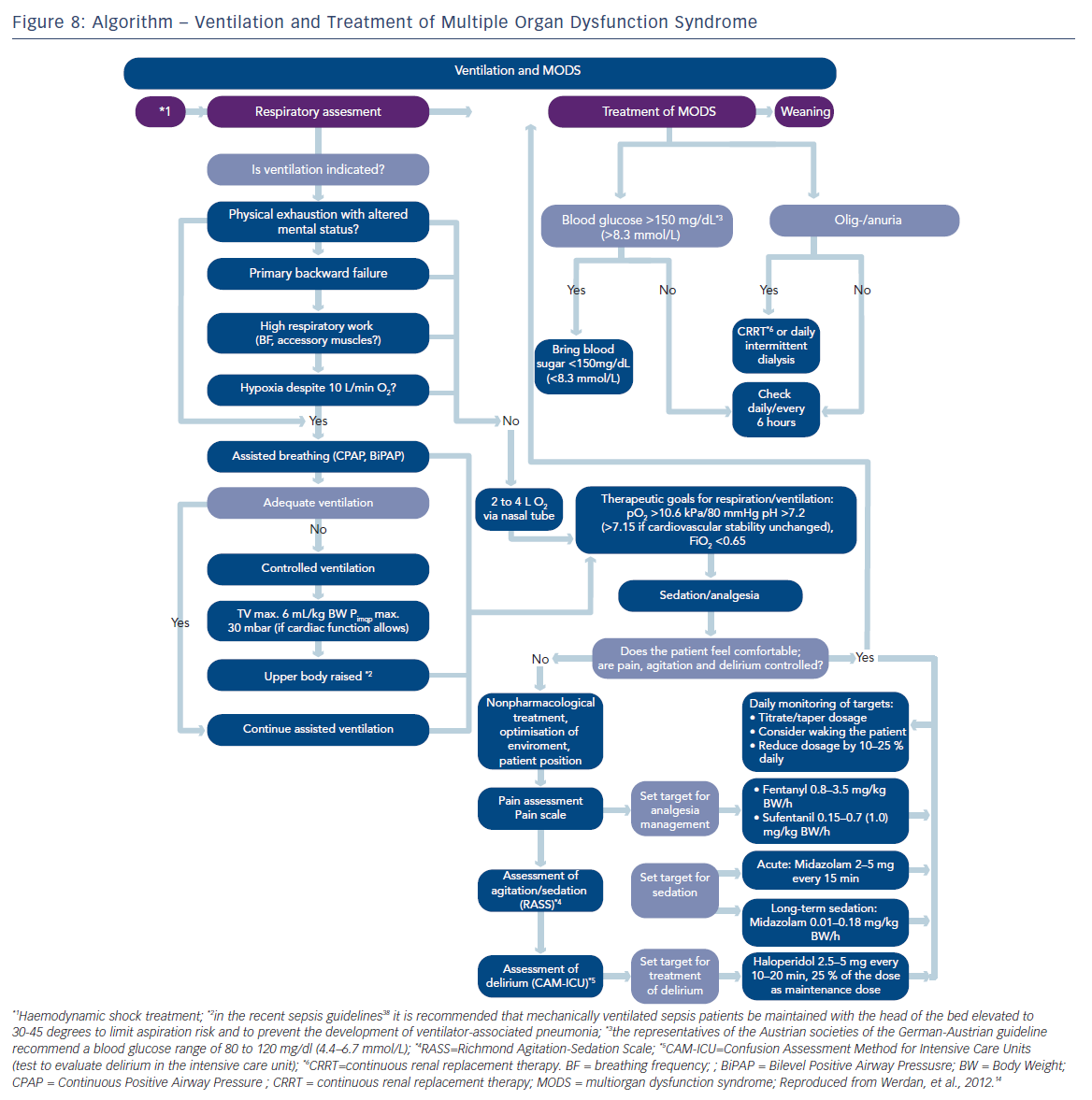
Updates of the European and American ST-elevation myocardial infarction (STEMI) guidelines including short chapters about cardiogenic shock (see Figure 2) have recently be published.6,13 As can be seen from Figure 2, these recommendations focus on coronary reperfusion as well as on cardiac and circulatory stabilisation, while general intensive care measures for prophylaxis and treatment of SIRS and MODS are not discussed in detail. The latter is the domain of the German-Austrian S3 guideline “Cardiogenic Shock Due to Myocardial Infarction: Diagnosis, Monitoring and Treatment” (condensed English version;14 German version15). This German-Austrian guideline with the highest guideline degree (S3) deals exclusively with cardiogenic shock complicating MI, with 111 recommendations (see Figure 3) and a total of seven algorithms, e.g. for ‘revascularisation’ (see Figure 4), for “hemdynamic shock therapy” (see Figure 5) and for “ventilation and treatment of MODS” (see Figure 8).
Early Diagnosis of Cardiogenic Shock – It is a Clinical Diagnosis
Cardiogenic shock after AMI develops within six hours in about 50 % of patients and within 24 hours in about 75 % of patients. Consequently, shock can even be present in the pre-hospital phase, and the emergency physician has to diagnose cardiogenic shock as soon as possible. Cornerstones of the diagnosis are the 12-lead electrocardiogram (ECG) (STEMI, seldom Non-ST elevation myocardial infarction [NSTEMI]), hypotension (systolic blood pressure <90 millimetres of mercury [mmHg] for at least 30 minutes [min] in the absence of volume depletion) and clinical signs of reduced organ perfusion (cold extremities, oliguria, altered mental status [e.g. agitation]). In those, one fourth of CSAMI patients without hypotension, diagnosis must exclusively rely on those clinical signs.14
Preclinical monitoring consists of blood pressure and heart rate, ECG, pulse oximetry, capnometry in case of mechanical ventilation and blood glucose measurement. Echocardiography as early as possible after arrival in the hospital can document systolic left ventricular dysfunction, mechanical infarct complications and right heart MI.15
Pre-hospital and initial hospital stabilisation of cardiovascular (dobutamine, norepinephrine) and pulmonary function (if necessary: mechanical ventilation) of the shock patient is mandatory to achieve optimal conditions for cardiac catheterisation.
Revascularisation
Emergency coronary revascularisation (usually PCI) shall be considered in suitable patients with cardiogenic shock due to pump failure after STEMI, irrespective of the time delay from MI onset (see Figures 2–4). Early coronary revascularisation is the best evidenced positive recommendation in cardiogenic shock guidelines. Though in the landmark Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial4 early revascularisation by either PCI or ACB showed only a trend to better 30-day survival in relation to conservative medical treatment (56.0 versus 47.6 %; p=0.11), survival after six months (49.7 versus 36.9 %; p=0.027), 12 months (46.7 versus 33.6 %; p<0.04) and six years (32.8 versus 19.6 %; p=0.03) all were significantly higher.2,4,16 One hundred and thirty-two lives can thereby be saved by every 1,000 patients treated.16
The detailed approach of revascularisation – as given by the German-Austrian guideline – is shown in Figure 4. PCI – the method of choice – is usually carried out with stent implantation, with intensive use of antithrombotic agents and intravenously applied unfractionated heparin (unclear pharmacokinetics in shock in case of subcutaneously applied heparin). PCI should be carried out within two hours after emergency contact. PCI is indicated in both genders and also – after individual risk–benefit judgment – in patients >75 years. When PCI fails, and also in specific situations (e.g. complex left main stenosis or complex multivessel disease) ACB should be done immediately. Whether in case of multivessel disease culprit lesion PCI only or complete revascularisation should better be carried out, is the topic of the ongoing Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial. This is a prospective, randomized, international, multicenter, openlabel study to compare immediate multivessel revascularization by percutaneous coronary intervention (PCI) to culprit lesion only PCI with staged non-culprit lesion revascularization in patients with cardiogenic shock complicating acute myocardial infarction presenting with multivessel disease. In contrast to STEMI patients without cardiogenic shock, no evidence-based data exist for CSAMI patients whether bare metal stents or drug-eluting stents should be favoured, about the role of the newer antithrombotic agents prasugrel and ticagrelor and whether thrombus aspiration is of benefit.
Resuscitated patients represent up to 30 % of CSAMI patients.17–19 In successfully defibrillated patients, early PCI should be considered and mild hypothermia (32–34 ° for 12–24 hours) should be applied (see Figure 3: E 78 and E 79/80).
All guidelines agree that systemic fibrinolysis should only be considered if revascularisation is unavailable (see Figures 2 and 4).

No specific fibrinolytic agent is recommended in CSAMI patients. The German-Austrian guideline15 additionally states (↑) that fibrinolysis should be undertaken before PCI when MI symptoms started <3 hours and PCI is not available within 90 min.
Blood Pressure Monitoring is Not Enough for Haemodynamic Shock Therapy
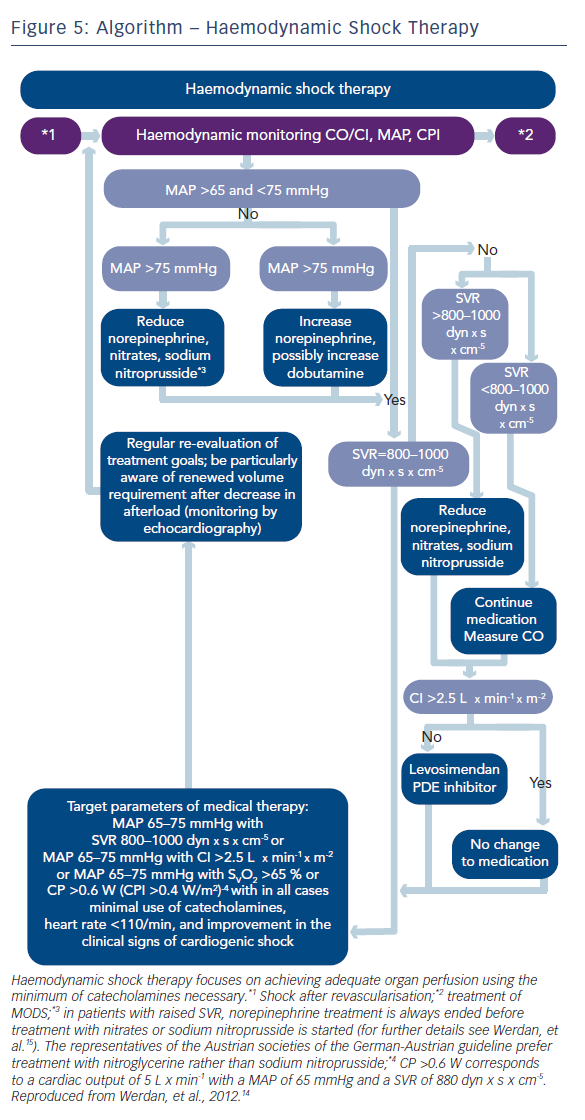
When shock symptoms persist after revascularisation, haemodynamic shock therapy has to be initiated (see Figure 5). An important question with this respect is how to guide this therapy. Usually, treatment is guided by blood pressure measurement.
However, stabilising blood pressure only cannot guarantee adequate organ perfusion. A better judgment of organ perfusion can be achieved when we include “flow measurement“ (i.e. cardiac output/cardiac index) in our haemodynamic monitoring. The best prognostic value of haemodynamic monitoring in cardiogenic shock is represented by a measure, which includes both pressure and flow,20,21 like the “cardiac power output“ (CPO; mean arterial pressure x cardiac output x 0.0022) and the “cardiac power index“ (CPI; mean arterial pressure x cardiac index x 0.0022).
Based on these findings, the German-Austrian guideline14,15 recommends monitoring of a combination of blood pressure and any of the flow equivalents like cardiac index, systemic vascular resistance (SVR), mixed venous oxygen saturation (SvO2)/central venous oxygen saturation (ScvO2) or the combined product CPO/CPI (Figures 3, E 32/33 and 5).
Which Inotrope, Which Vasopressor?
While the European guidelines6 recommend dobutamine as well as dopamine as inotrope, the German-Austrian guideline14 clearly favours dobutamine (2.5 μg x kg-1 x min-1) (Figures 3, E 34-38 and 5), based on findings of a multicentre cohort observational study with 1,058 shock patients,22 in which application of dopamine was an independe nt risk factor for mortality, while dobutamine and norepinephrine were not.
There is guideline agreement6,14 that norepinephrine (0.1–1.0 μg x kg-1 x min-1) is the vasopressor of choice in favour of high-dose dopamine when blood pressure is low (Figures 2 and 3, E 34–38 in Figure 5). The evidence comes from the Sepsis Occurrence in Acutely Ill Patients (SOAP) II study with 1,679 patients with shock of various aetiologies.23 In the SOAP II study, norepinephrine showed a tendency to lower 28-day mortality compared with dopamine (45.9 versus 50.2 %, 95 % confidence interval [CI] 0.98–1.44; p=0.07), which was significant in the prospectively defined subgroup of patients with cardiogenic shock (odds ratio [OR] 0.75; p=0.03). Furthermore, significantly fewer arrhythmias (12.4 versus 24.1 %) occurred in the norepinephrine group than in the dopamine group of the SOAP II study, especially atrial fibrillation.
In catecholamine-refractory cardiogenic shock after AMI, the German- Austrian guideline14 favours – instead of a phosphodiesterase 3 inhibitor – the additional treatment with the inodilator levosimendan, with – cave: hypotension – or without a bolus of 12–24 microgram/kg/10 min, followed by 0.05–0.20 μg/kg/min for 24 hours (E 34–38 in Figure 3). This recommendation is based on the results of a small randomised trial with levosimendan versus enoximone.24 Shock patients under pre-existing chronic beta-blockers could possibly be stabilised better by levosimendan or by phosphodiesterase 3 inhibitors as by dobutamine, in analogy to findings described in patients with acute decompensated heart failure.25,26
Intra-aortic Balloon Pump Counterpulsation Treatment of Cardiogenic Shock After Acute Myocardial Infarction – Disappointing Results
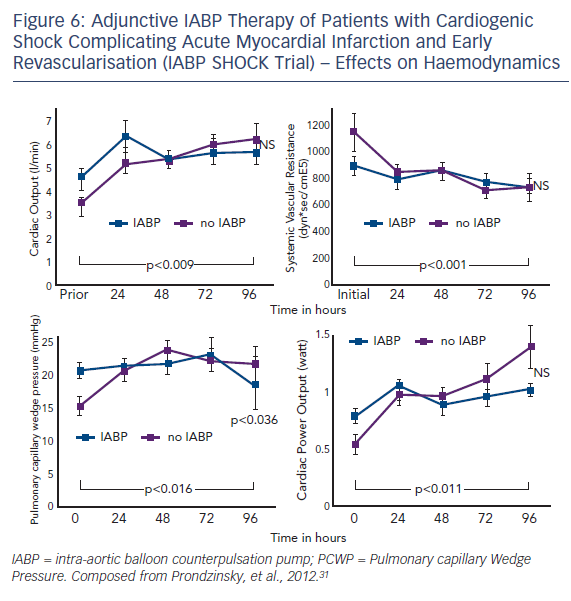
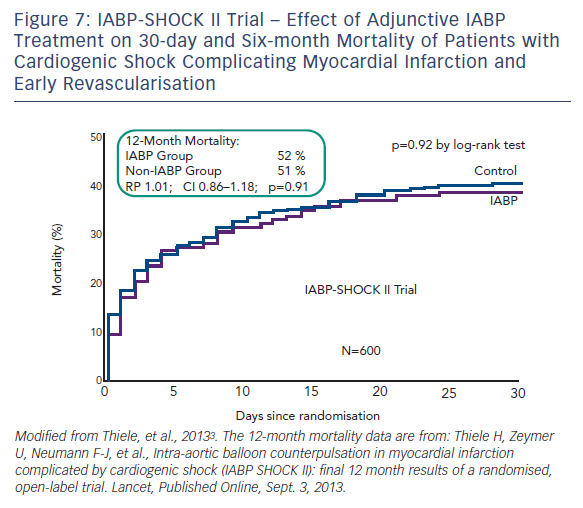
Intra-aortic balloon counterpulsation pump (IABP) is widely used for the treatment of acute and chronic cardiac decompensation, though the evidence is scarce.27,28 While until recently, IABP implementation was a class I recommendation of the European as well as the American STEMI guidelines, doubt was cast on the benefit of IABP in CSAMI patients by the negative results of a meta-analysis of 10,529 patients from nine cohort studies29 and a recent Cochrane analysis on six randomised studies with 190 patients.30 In the former,29 patients treated with the recommended standard therapy (PCI) had no additional benefit from adjunctive IABP treatment; only those treated with systemic fibrinolysis showed an additional reduction in mortality by 18 % by adjunctive IABP therapy. Furthermore, in the randomised Intraaortic Balloon Pump in cardiogenic shock (IABP SHOCK) trial with 40 patients treated with early revasculariation, adjunctive IABP therapy did not result in a significant haemodynamic improvement compared with standard treatment alone (see Figure 6), with the exception of a transient lowering of plasma BNP levels in the IABP group.11,31 And finally, in the randomised IABP SHOCK II trial (n=600) neither a reduction of 30-day nor of 12-month mortality could be achieved by adjunctive IABP treatment in addition to early revascularisation (see Figure 7).3
All guidelines drew consequences from these disappointing results (see Figures 2 and 3): now the European STEMI guidelines6 rank IABP implementation in cardiogenic shock complicating MI only as a class IIb recommendation with level B evidence, and the American STEMI guideline13 gives adjunctive IABP therapy now a class IIa recommendation with level B evidence. The German-Austrian S3 guideline14 gives a weak recommendation for adjunctive IABP therapy only for those patients treated with systemic fibrinolysis (E 44 in Figure 3), and tells that in patients with early revascularisation IABP implementation can be considered but knowing that the available evidence is unclear (E 45 in Figure 3). Furthermore, the German-Austria guideline gives a weak recommendation for IABP therapy during intrahospital transport for early revascularisation of shock patients and in case of mechanical infarct complications for pre-operative stabilisation.15
Alternative percutaneous left ventricular assist devices (LVADs) – centrifugal pumps without oxygenator (Tandem Heart™) and with oxygenator (venoarterial extracorporeal membrane oxygenation [ECMO], LifeBridge®) as well as axial flow pumps (Impella Recover LP®2.5 and Impella Recover LP®5.0) are haemodynamically more effective than the IABP. In retrospective analyses and case series, encouraging results were reported, but randomised controlled trials in CSAMI patients are still lacking.28,32–34 Two meta-analyses show better haemodynamic improvement by LVADs (TandemHeart and Impella) than by IABP, but no better outcome (30-day mortality) and more complications.30,35 Both, the European as well as the American STEMI guidelines rank the use of LVADs in patients with refractory CSAMI as IIb/C (see Figure 2). The German-Austrian guideline15 does not give any recommendations for the use of LVADs in CSAMI patients.
Do Not Disregard the Right Mode of Ventilation
In cardiogenic shock, work of breathing consumes a considerable part of the strongly reduced cardiac output, leading to further impairment of perfusion of vital organs. Mechanical ventilation guarantees oxygenation and reduces the work of breathing. If ventilation is necessary in CSAMI patients, especially in backward failure (see Figure 8), then invasive ventilation is preferred over non-invasive ventilation (E 86 in Figure 3). According to the German-Austrian guideline, “the reasons for this are the constant stable ventilation conditions provided by invasive ventilation and the avoidance of psychomotor excitement that would exhaust the patient”.14
After having achieved sufficient oxygenation during the initial phase of mechanical ventilation, then the question of best ventilation mode arises. In adult respiratory distress syndrome (ARDS), this is undoubtedly lung protective ventilation, limiting tidal volume to <= 6 ml/kg of predictive body weight and peak inspiratory pressure up to 30 millibars (mbar). Although in patients with CSAMI information about ventilation36 is sparse with this respect, the experts of the German-Austrian guideline recommend lung-protective ventilation also in CSAMI patients, if tolerated from a cardiac point of view (E 88 in Figure 3 and 8). A retrospective monocentric analysis of 129 mechanically ventilated patients with cardiac failure supports this recommendation:37 14.7 % of these patients with a hospital mortality of 47.3 % had lung protective ventilation on day one, with a significantly higher proportion in survivors than in non-survivors (24.1 versus 9.6 %; p<0.05).
Standardised ventilation and weaning is essential (E 93,94 in Figures 3 and 8): “The depth of analgesia/sedation should be recorded three times a day using the Richmond Agitation–Sedation scale. Weaning, which is often difficult in cardiogenic shock after AMI patients, should follow a weaning protocol, and before weaning starts, the following conditions should be fulfilled: hemodynamic stability, absence of myocardial ischemia, and absence or regression of inflammation or infection“.14,15,38
Specific Shock Forms After Acute Myocardial Infarction
These include shock following right ventricular infarction and shock due to mechanical AMI complications (mitral regurgitation due to papillary muscle dysfunction or rupture, ventricular septal defect and pericardial rupture).6,15 Important in case of shock following right ventricular infarction is revascularisation as early as possible, an adequate increase in right ventricular preload (central venous pressure 15–20 mmHg) with careful watching of left ventricular dysfunction, increasing right ventricular inotropy with dobutamine and – in case of bradycardia – acutely given atropine and eventually pacing. Treatment of choice of shock due to mechanical AMI complications usually is early cardiac surgery, with pre-operative haemodynamic stabilisation by IABP implementation.
Complications of Cardiogenic Shock After Acute Myocardial Infarction
Arrhythmias
Arrhythmias in the course of acute myocardial infarction6 and especially in CSAMI patients15 – either pre-existing, triggering cardiogenic shock or as a consequence of acute heart failure (6–32 %)39 – can further aggravate cardiac dysfunction. Electrical cardioversion/defibrillation and amiodarone are therapies of choice in case of haemodynamically relevant recent onset atrial fibrillation and ventricular tachycardias.15
Multiple Organ Dysfunction Syndrome
The cardiogenic shock – especially if prolonged – triggers a drastic hypoperfusion of vital organs with the consequence of development of SIRS (see below) and MODS. Consequently best prophylaxis of MODS development and best therapy of MODS is rapid restoration of a sufficient organ perfusion.
Scores like APACHE II (see Figure 1)/III, SAPS II and SOFA characterise the severity of MODS, which is an important determinant of mortality (see Figure 1).11,40 Concerning the organs involved in MODS, the lungs (ARDS) play a prominent role (see above). Treatment of acute renal failure can be compensated by either continuous renal replacement therapy (CRRT) or by intermittent haemodialysis, with a weak recommendation (↑) for CRRT because of better tolerability in haemodynamically unstable patients.15 Other organ dysfunctions consist those of the gastrointestinal tract and the liver, the critical illness neuropathy, myopathy, encephalopathy and autonomous dysfunction as well as the endocrine and the metabolism.15 Despite the postulated relative adrenal insufficiency in shock, no convincing data exist about a beneficial role of low dose hydrocortisone in CSAMI. No information is available for CSAMI about the role of selective decontamination of the digestive tract (SDD), which may be useful in septic shock.38
Systemic Inflammatory Response Syndrome
Patients with CSAMI have increased serum levels of pro-inflammatory mediators like IL-6 (see Figure 1), C-reactive protein and many others,8,9 even as high as in septic shock. This clearly indicates a high state of SIRS in these patients, which is of prognostic relevance. Consequently, the TRIUMPH (The Tilarginine Acetate Injection in a Randomized International Study in Unstable MI Patients With Cardiogenic Shock) trial41 tested tilarginine (L-NG-monomethylarginine [L-NMMA]), a non-selective nitric oxide synthase (NOS)-inhibitor in 398 patients with MI and refractory cardiogenic shock despite an establishment of an open infarct artery. Tilarginine did neither reduce 30-day mortality in comparison with placebo (48 versus 42 %; risk ratio 1.14; 95 % CI 0.92–1.41; p=0.31), six-month mortality nor improve any other morbidity measure. At present, no effective specific anti-inflammatory therapy is available in CSAMI patients.
Sepsis, Severe Sepsis and Septic Shock
In one of every six CSAMI patients, sepsis/severe sepsis with organ failure or septic shock develops during the first days of shock development.7 Positive blood cultures revealed Staphylococcus aureus (32 %), Klebsiella pneumoniae and Pseudomonas aeruginosa as the dominant pathogens, particularly in patients with prolonged IABP support or multiple central catheters.42 In the SHOCK trial, septic patients had an inadequately low SVR for patients with cardiogenic shock (1,051 [862–1,486] versus 1,402 [1,088–1,807] dyn × s × cm-5) and a twofold higher mortality.7
To diagnose sepsis as early as possible, careful monitoring of clinical and haemodynamic sepsis signs in these patients is mandatory; and if sepsis is suspected or is confirmed by increased procalcitonin levels (cut-off 2 ng/ml), blood cultures should be immediately drawn and sepsis therapy immediately started according to the sepsis guidelines – according to the three hour sepsis bundle, within three hours the following should be completed:38
- measure lactate level;
- obtain blood cultures prior to administration of antibiotics;
- administer broad spectrum antibiotics (within one hour); and
- administer 30 mL/kg crystalloid for hypotension or lactate 4 mmol/L.
Especially in CSAMI patients, fluid administration must be carefully monitored and titrated.
Intensive Care for the Patient with Cardiogenic Shock After Acute Myocardial Infarction
Nutrition – Enteral Nutrition is Preferred!
Enteral nutrition is preferred over parenteral nutrition. Critically ill patients of 20–30/30–70/>70 years in the catabolic acute phase need a daily caloric intake of no more than 25/22.5/20 kcal/kg, with the shock stage even decreasing energy turnover. Hyperalimentation of the CSAMI patient of >25 kcal/kg/day should be avoided.15
Blood Glucose – Keep a “Middle Way”!
The initially propagated beneficial effect of normalising blood glucose levels (4.4–6.1 mmol/L / 60–110 mg/dL) by continuous insulin infusion in intensive care patients could not be confirmed in later studies as the NICE-SUGAR (Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation) study,43 which also pointed to the risk of hypoglycaemia with this concept. On the other hand, high blood glucose levels in heart attack patients indicate an unfavourable prognosis.44 As compromise, the German-Austrian guideline14 gives a weak recommendation (↑) to keep blood glucose values below 150 mg × dL-1/<8.3 mmol × L-1 by means of insulin (E 99 in Figure 3 and 8). The formerly used glucose-insulin-potassium infusions given to AMI patients show no benefit in AMI patients44 and shall not be given to CSAMI patients (E 100 in Figure 3).
Which Haemoglobin Threshold Can be Accepted in Cardiogenic Shock After Acute Myocardial Infarction Patients?
A lot of controversy does exist at what haemoglobin (Hb) threshold red blood cells should be transfused in intensive care patients, especially in those with cardiac disease and in older patients in whom increased oxygen requirement of the heart may be assumed.15 Based on intense discussions of the experts, the German-Austrian guideline gives the weak recommendation (↑) that red cell concentrate transfusions should be given in CSAMI patients when haemoglobin values are <7.0 g × dL-1 /4.3 mmol × L-1 or haematocrit <25 % and the values be brought up to 7.0–9.0 g × dL-1/4.3–5.6 mmol × L-1 or ≥25 % (E 101 in Figure 3); in older patients with CSAMI a fall of the haematocrit below 30 % should be avoided (E 102).15
Mandatory Prophylactic Measures
Thrombosis prophylaxis with heparin shall be given by the intravenous route, at least during shock phase (see above) (E 103/104).15 As most CSAMI patients have risk factors for gastroenteral bleeding, stress ulcer prophylaxis, mostly done with proton pump inhibitors,45 should be applied (E 107).15
Bicarbonate is often used in shock patients with blood acidosis. However, no proven benefit was achieved in two studies with blood acidosis not lower than 7.15,46,47 but with possible harm (retention of sodium ion [Na+] and fluid, increase in lactate and carbon dioxide partial pressure [pCO2], as well as a fall in ionized serum calcium). Therefore, the German-Austrian guideline recommends (↑) that bicarbonate should not be used for treatment of hypoperfusion-induced lactic acidosis with a blood pH ≥7.15 with the intention to stabilise haemodynamics or reduce vasopressors (E 108).15
What About Guideline Adherence Under Cardiologists Treating Patients with Cardiogenic Shock After Acute Myocardial Infarction?
We all know that guideline knowledge is no guarantee for guideline adherence.48,49 With respect to the treatment of CSAMI patients, we can be sure that early revascularsation – a class I recommendation of all guidelines6,13,14 has a very high adherence, and the recommendation of the German-Austrian guideline14 of lung protective ventilation a low one37 (see above). However, systematic research on this topic is scarce in CSAMI patients.
In the IABP SHOCK II trial having included 600 patients with CSAMI,3 treatment was standardised according to the German-Austrian S3 guideline,14,15 which was provided to the investigators of all 37 participating centres located in Germany. Guideline adherence in this trial is presently under investigation.
Limitations of Evidence-based Management of Cardiogenic Shock after Acute Myocardial Infarction
Most of the given guideline recommendations are based on low evidence levels (see Figures 2 and 3): results from non-randomised trials, expert opinions and recommendations deduced from guidelines for noncardiogenic shock ICU patient groups. Only a few recommendations are based on data from large randomised trials or meta-analyses, like the benefit of early revascularisation and the neutral result of IABP therapy. A lot of work will have to be done in the future, to give evidence-based management of cardiogenic shock a better fundament. This should include standardised and consented nursing guidelines for these patients.
Though hospital mortality of CSAMI patients is very high, long-term prognosis of the surviving patients is encouraging – after one year, more than half are in New York Heart Association (NYHA) class I or II,16,50 and many are completely asymptomatic.51 Optimisation of postintensive and rehabilitation care could help to improve prognosis and quality of life of these patients even further.