Transcatheter aortic valve implantation (TAVI) has emerged as a safe and effective strategy for the treatment of symptomatic and severe aortic valve stenosis (AS).1 The choice between a transcatheter or surgical approach is no longer dependant on the estimated surgical risk, but rather the institutional heart team’s assessment of medical comorbid illnesses and frailty, individual cardiac and vascular anatomic characteristics, the patient’s preferences and local experience. The major advantages of TAVI with regard to surgical aortic valve replacement (SAVR) include a less invasive approach with rapid recovery and lower risk of short-term death and stroke.1 SAVR, in contrast, is less dependent on vascular, aortic root or valve anatomy, and is associated with a lower risk of post-procedural paravalvular leak or the requirement for a permanent pacemaker.1 In addition, TAVI presents a risk for acute or subacute coronary artery obstruction (CAO), a rare but devastating and life-threatening complication. Coronary artery protection with an option to perform ‘chimney stenting’ is an important and ever more frequently used technique that can mitigate against CAO during TAVI. In this article, we describe the risk factors associated with CAO, the procedural steps to perform coronary protection and chimney stenting and discuss the available literature on this topic.
Coronary Artery Obstruction in Transcatheter Aortic Valve Implantation
Acute CAO is defined as a new complete or partial obstruction of one or both coronary ostia during a TAVI procedure.2 This complication typically manifests as abrupt haemodynamic instability with rapid progression to cardiogenic shock and ventricular arrhythmias.
The incidence of acute CAO during TAVI in native aortic valves is relatively low (<1%) but occurs three- to fourfold times more frequently after valve-in-valve (VIV) procedures (2.3–3.5%).3–6 Obstruction of one or both coronary arteries is usually caused by direct coverage of the ostia by displaced (bulky) native leaflet tissue that is pushed aside by the frame of the expanded transcatheter heart valve (THV). In case of VIV procedures, it is the displacement of the bioprosthetic leaflet tissue that can cover the coronary ostia. Displaced leaflets, native or prosthetic, can also reduce coronary flow towards the sinus of Valsalva (SOV) when pushed into contact with the sinotubular junction. It is rather uncommon that components of the THV itself (e.g. skirt, commissural posts) directly obstruct the coronary ostia; however, this should be suspected in the setting of shallow aortic sinuses. Coronary dissection, haematoma or embolisation of thrombotic or degenerative material are alternative, less-common mechanisms of CAO.
Acute CAO has a major effect on morbidity and mortality after TAVI. The 30-day mortality of acute CAO is high, ranging between 8% and 41% for TAVI in native AS and up to 53% in cases of VIV.3,4,6 The type of coronary revascularisation, and hence the rapidity of restoration of coronary blood flow, appears to be an important determinant of outcome after CAO. A multicentre registry reported a 30-day mortality of 22% in patients successfully treated with percutaneous coronary intervention (PCI), 50% in patients who were treated with urgent coronary bypass grafting (CABG) and a striking 100% 30-day mortality among patients with unsuccessful PCI.3
Recently, delayed CAO has been reported as a rare cause of MI after TAVI, associated with similarly high in-hospital mortality rates (50%).7 In these cases, CAO occurs after the patient has left the catheterisation laboratory after TAVI and is categorised as either early (0–7 days) or, less frequently, late (>7days) presentation after the index procedure.7 Clinicians should be aware that similar risk factors as for acute CAO are associated with the early type of delayed CAO. Further stent expansion or thromboembolic phenomena occurring in shallow and overfilled SOV are presumed factors for the occurrence of early delayed CAO. In cases of late delayed CAO, occurring months to years after the procedure, mechanisms related to endothelialisation, fibrosis and thrombosis are presumed to cause this late complication.
Risk Factors for Acute Coronary Artery Obstruction
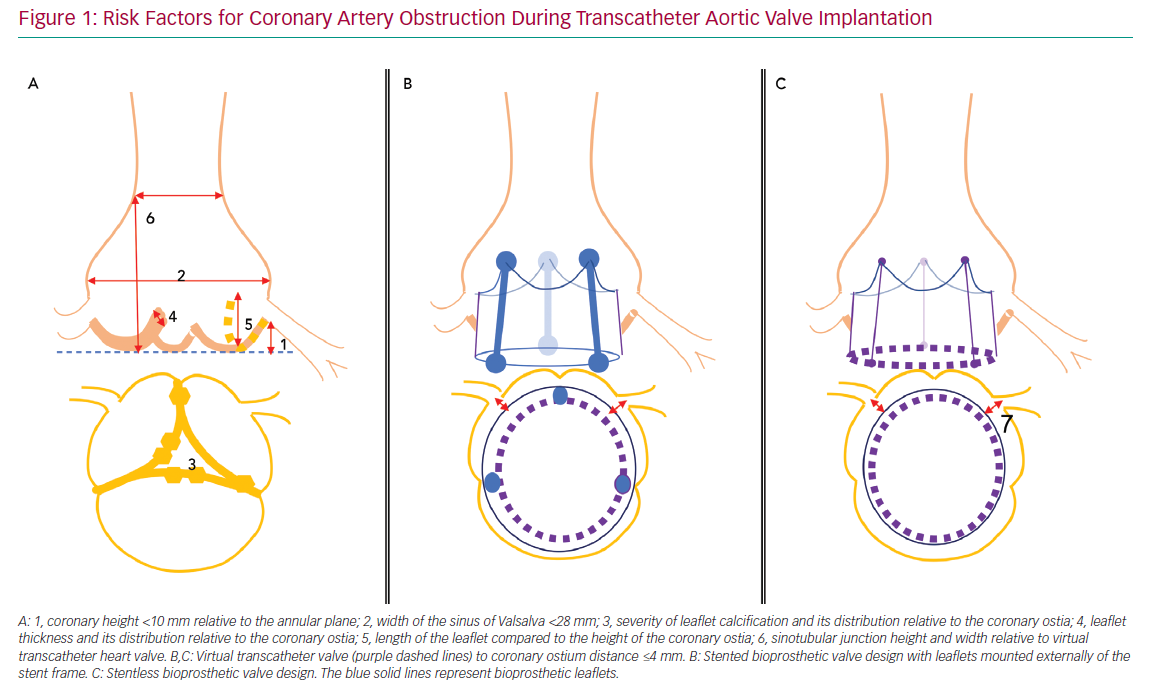
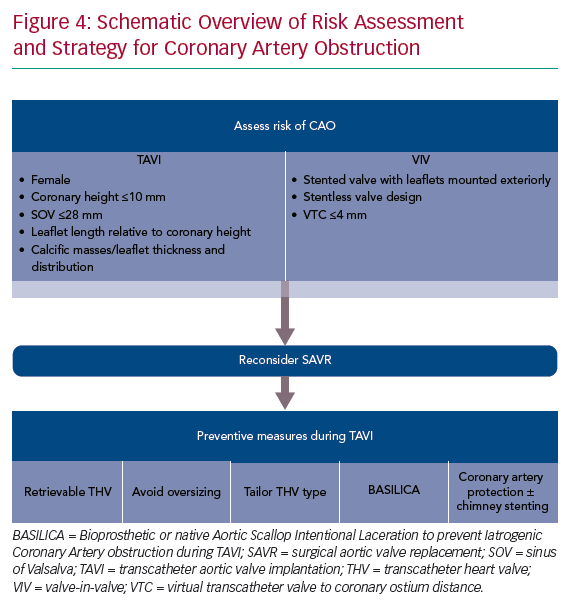
Risk factors for acute CAO during TAVI have been identified and include female sex, coronary height <10 mm from the annular plane and SOV <28 mm.3,4,8 However, a systematic review of acute CAO cases reported between 2002 and 2012 showed approximately 60% with left coronary artery (LCA) height >10 mm, and most patients with acute CAO in observational trials had an LCA height of <12 mm or SOV <30 mm.3,4 These data suggest higher cut-off values may be more accurate, but also that other risk factors may be involved. Potential adverse anatomical features that should be assessed during preprocedural planning include the severity of valve calcification, the distribution of bulky calcifications and thickened leaflet tissue, the length of the native leaflets relative to ostial height of the coronaries or the height and width of the sinotubular junction.
Not surprisingly, in the available registries, patients with CAO less frequently had a history of CABG.3,4 At the same time, this may be cause of underreporting of the true prevalence of CAO in these registries, because CAO symptoms may have been obscured by ‘graft protection’.
For VIV procedures, additional risk factors include the treatment of surgical bioprostheses with externally mounted leaflets, stentless bioprosthetic valves, patients with a virtual transcatheter valve to coronary ostium distance <4 mm, or those with a virtual transcatheter to sinus tubular junction distance <4 mm (Figure 1).6
Strategies in At-risk Patients
When patients are identified to be at an increased risk of CAO during TAVI, a robust discussion in the institutional heart team is essential. In such cases, SAVR may be a more appropriate treatment strategy. In cases where a surgical approach is not feasible, several procedural techniques can be considered to reduce the risk of CAO during TAVI.
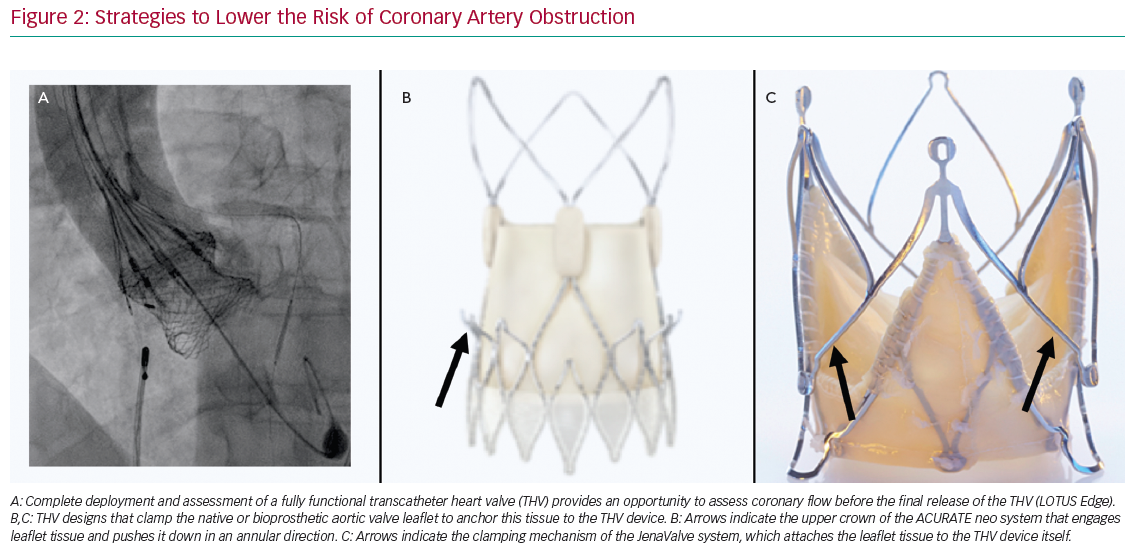
Some observational data suggest a higher risk of CAO with balloon-expandable (BE) than self-expandable (SE) THVs.3,4 Although it has been suggested this may be explained, in part, by differences in frame characteristics, it should be mentioned that the manufacturer of SE CoreValve rules out specific recommendations regarding SOV diameter and coronary height, with these recommendations not provided by the manufacturer of the BE Edwards valves. Therefore, it is not generalisable that SE THVs should be preferred above BE THVs in patients at risk of CAO. Conversely, a recapturable THV system provides the advantage of assessing CAO before final deployment of the valve. For example, the Lotus Edge (Boston Scientific) THV allows thorough assessment of implantation position, function, sealing and relationship to the coronary arteries prior to release. If malposition or CAO is observed, the prosthesis can simply be recaptured and repositioned or removed (Figure 2A). No other currently commercially available THV system can be assessed while fully deployed and in position before final release. Other THVs have been designed to clamp native or bioprosthetic aortic valve leaflets to ‘anchor’ this tissue to the THV device and potentially reduce the risk of CAO. For example, the ACURATE Neo (Boston Scientific) THV system is designed such that the upper crown ‘engages’ leaflet tissue and pushes it down in the direction of the annulus (Figure 2B). A trial in 30 patients at high risk of acute CAO (mean ± standard deviation left main height 10.8 ± 1.5 mm and shallow SOV with an SOV:annulus ratio 1.8 ± 0.8 mm) showed no acute CAO after transapical implantation of the ACURATE Neo THV system.9 The JenaValve (JenaValve Technology) is another second-generation THV that has a clipping mechanism to grasp the native leaflets and attaches them to the THV device (Figure 2C). Data on the rate of CAO with the most recent version of the JenaValve system are not yet available.
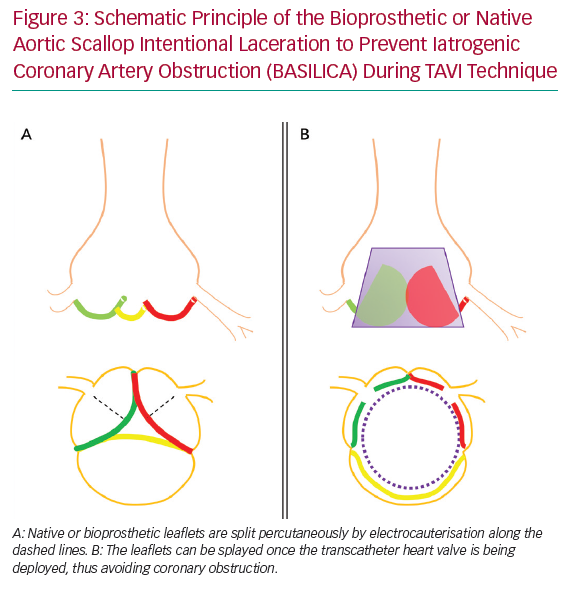
Bioprosthetic or Native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction (BASILICA) during TAVI is a contemporary method to decrease the risk of acute CAO. In this technique, native or bioprosthetic leaflets that risk CAO are intentionally sliced using transcatheter electrocauterisation. This enables the leaflets to splay once the THV is being deployed, and hence maintain blood flow into the SOV and coronaries (Figure 3).10 A prospective single-arm study in 30 patients (57% VIV) at high risk of CAO demonstrated this technique to be successful in 93% of patients, with no cases of CAO or reintervention after 30 days.11 Three patients (10%) had neurological events (one disabling stroke, two non-disabling stroke). In 43% of patients, cerebral protection was provided. This procedure was tolerated haemodynamically in most patients (93%). In cases when haemodynamic instability appeared, this resolved immediately after completion of THV deployment. Primary reports appear to show acceptable safety of BASILICA, but this needs confirmation in larger trials. This technique is currently not widely available outside expert centres.
Despite these aforementioned strategies to avoid CAO, patients at risk of CAO continue to present for TAVI. In such cases, prophylactic coronary protection with a wire, balloon or stent should be considered as a facilitatory step in case bailout ‘chimney’ or ‘snorkel’ stenting is required to manage acute CAO. This technique has been successfully described in case reports and small case series.12–14 A coronary balloon or stent premounted on the protective 0.014" guidewire can be parked distally in the coronary artery, retrieved proximally and deployed with rapid restoration of coronary flow in case of acute CAO. We consider pre-emptive coronary protection in all at-risk patients as described in Figure 4.
Coronary Protection and Chimney Stenting
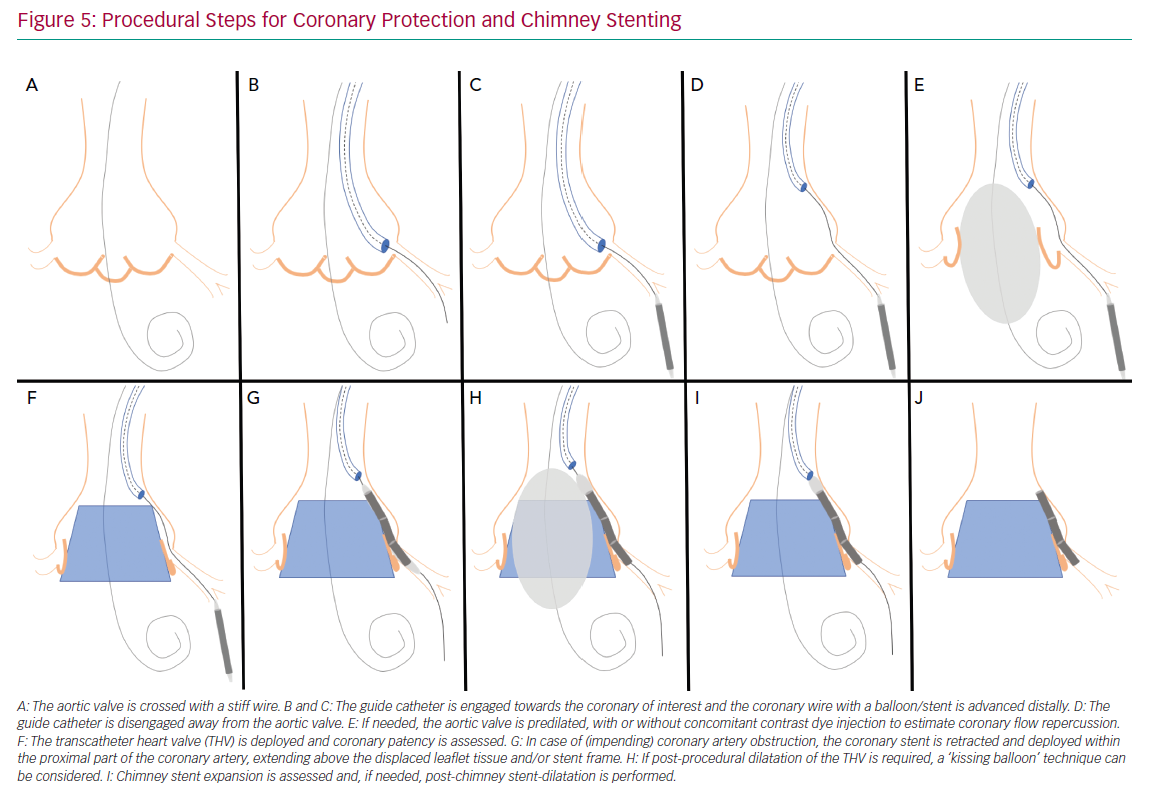
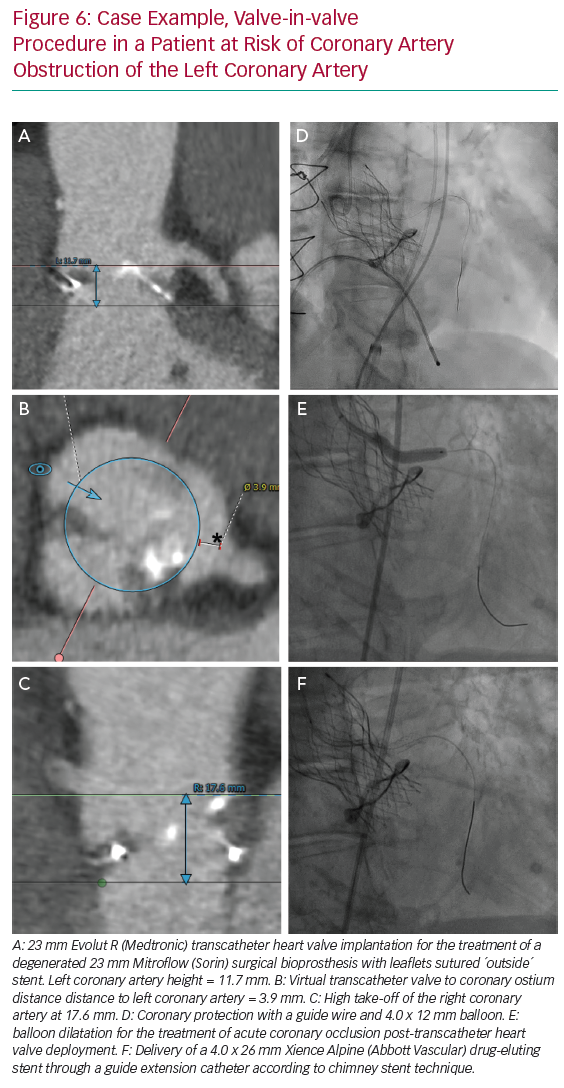
Figure 5 illustrates the steps used for coronary protection and chimney stenting, and a case example is shown in Figure 6. Coronary artery protection during TAVI usually necessitates the use of an additional arterial access for guide catheter engagement of the at-risk coronary artery. Alternatively, the retracted guide catheter can be used for contrast dye injections while deploying the THV, in lieu of a pigtail catheter and subsequently avoiding an extra arterial access.15 Ideally, Judkins left/right or multipurpose guiding catheters are chosen because they are easier to back up into the aorta during THV deployment and can be repositioned towards the coronary ostium once the THV is deployed. Other guiding catheters can also be used successfully (Ikari, EBU or AL1), but may be more difficult to reposition after THV implantation. Because of the risk of haemodynamic instability after balloon aortic valvuloplasty (BAV), we prefer to have the coronary protection kit in situ prior to BAV. The coronary guide catheter should be engaged, with delivery of a coronary guide wire distal in the vessel under appropriate heparinisation (activated clotting time >250 s). The guide catheter is then retracted into the ascending aorta once a premounted balloon or stent over the protective wire is positioned distal in the coronary vessel. The decision to premount a balloon or stent on the coronary guide wire depends on the anatomical characteristics of the aortic root and the perceived risk of CAO. Although a guide wire-only strategy saves time and money, it can be difficult to advance a coronary stent alongside the deployed THV (and displaced native leaflets) due to obstructing calcification or jailing of the safety wire between the aortic wall and the THV frame. In two observational trials, a 10–20% rate of failure to deploy a stent occurred in cases where stenting was attempted for the treatment of CAO during TAVI.3,4 A more recent registry of patients who underwent chimney stenting for established or impending CAO showed that the absence of a coronary protective wire was associated with increased rates of death, cardiogenic shock or MI.14 It is therefore suggested that a protective coronary guide wire with a premounted coronary stent is positioned distally in the coronary vessel prior to THV deployment.
If acute CAO is evident after BAV or THV deployment (i.e. chest pains, reduced coronary blood flow, ST-segment changes, ventricular arrhythmias, haemodynamic instability), immediate restoration of coronary flow is crucial and the parked balloon/stent can be deployed as described below. If, however, there is no clinical evidence of acute CAO but the angiographic appearance is suspicious for impending CAO (e.g. evidence of leaflet tissue directly in front of the coronary ostium with or without reduced coronary flow), coronary stenting should still be considered. Indeed, the presence of a coronary protection wire can provide false reassurance that CAO is not imminent by keeping displaced leaflet tissue away from the coronary ostium and maintaining normal coronary blood flow. In such cases, withdrawal of the wire can precipitate acute CAO and readvancement of the wire can be challenging. Moreover, a recent report on delayed coronary obstruction after TAVI reported that 23.7% of cases of delayed CAO had coronary protection during their index TAVI procedure.7 Conversely, a retrospective registry that included 93 at-risk patients who had a coronary protective wire but no final coronary stenting during TAVI showed a considerable risk (4.3%) for definite delayed CAO and demonstrated a high mortality rate in those patients (three of four with a fatal outcome).16 Intravascular ultrasound assessment of the coronary ostium and the adjacent aortic sinus can be an additional tool in such cases to determine the proximity of displaced leaflet tissue or calcifications to the coronary ostium, especially when there is doubt as to the need for stenting based on angiographic images only.12,16,17 A low threshold for stent deployment should be contemplated in these high-risk patients. Conversely, if there are no suggestions for impeding coronary flow or CAO after THV deployment, the coronary stent can be cautiously retracted.
If established or impending CAO occurs, the parked stent can simply be retracted and deployed. The coronary stent width should be selected according to the preprocedural CT analysis or angiographic assessment. The length of the stent length should be long enough so it has sufficient length to anchor in the proximal portion of the coronary artery and extend above the anticipated obstructive factor. If CAO is expected to be caused by displacement of bulky leaflet tissue, the stent length should be adjusted to extend above these obstructive leaflets; alternatively, if it is anticipated that the CAO is caused by closure of the entire sinus due to contact between the THV frame and the sinotubular junction, the stent chosen should extend above the sinotubular junction. Alternatively, the stent can be positioned at the level of the coronary ostium, while the THV is being deployed. This latter strategy can be used when a very high risk of CAO is anticipated and the risk of stent displacement or guide wire loss during THV deployment is low, or if there are concerns for the development of ischaemia with the stent placed deeper in the coronary artery.
After the stent is retrieved to the desired position, it should be inflated to high pressure (12 atm). The stent balloon can then be partially retracted for repeat inflation at higher pressure in order to flare the proximal portion of the stent. Importantly, the deflated stent balloon should not be withdrawn out of the coronary stent frame before THV function has been assessed because, in case post-dilation of the THV is required, a ‘kissing balloon’ technique can be used to avoid crushing of the chimney stent, as re-engagement of the balloon into the chimney stent can be challenging (Figure 5H). Compression or recoil of the implanted coronary stent by the expanded THV and displaced native leaflets has been reported.4,16 In such cases, a second stent can be implanted to improve stent expansion. Here, intravascular imaging can facilitate decision making if any further optimisation of the chimney stent(s) is needed.
Long-term Outcome of a Chimney Stent
Some specific concerns regarding long-term outcomes of chimney stenting need to be recognised. First, in a milieu of turbulent flow and calcific debris, a stent protruding far into the aorta that has not been apposed to the coronary artery wall over a length of several millimetres could, theoretically, be at considerable higher risk of chimney stent failure, including thrombosis or restenosis. Second, there are no available data to guide the intensity or duration of antiplatelet therapy. This requires careful consideration in patients who have an increased bleeding risk due to their comorbidities. Individualisation of antiplatelet therapy is mandatory, with most centres suggesting 3–9 months of dual antiplatelet therapy. The third concern relates to future access to the coronary circulation for routine management of stable or unstable coronary artery disease. This access is expected to be extremely challenging in this patient cohort. One study suggested easier reaccess in BE THV systems because the THV frame, and subsequently the chimney stent length protruding in the aorta, are both shorter.14
To date, no long-term follow-up or data on systematic imaging with coronary CT or angiography of chimney stents are available. However, recently the results of two retrospective registries have been published, collecting data on patients who received chimney stenting with clinical follow-up for up to 3 years.14,16 In the International Chimney Registry, a retrospective observational trial collecting data on 60 patients who were treated with chimney stenting for established or impeding CAO, there was a stent failure rate of 5.3% after a median follow-up of 612 days (interquartile range 405–842 days).14 Data on 236 at-risk patients undergoing TAVI with coronary protection wire were collected in the Coronary Protection to Prevent Coronary Obstruction During TAVR (COPROTAVR) registry.15 In that trial, 143 patients (60.6%) received coronary stenting (79% chimney stenting, 21% ostial stenting). After a 3-year follow-up, clinical outcome was generally favourable in patients treated with stenting (cardiac mortality 7.8%, MI 9.8%, stroke 5.4%). Although the occurrence of stent thrombosis was low (0.9%), it was fatal in all cases. No cases of in-stent restenosis were observed. In the group of stented patients, in three of four patients with MI who underwent angiography no coronary artery disease could be identified. It is not clear whether an underlying thromboembolic source, triggered by the protruding stent, for this latter finding should be considered. These trials are the first to suggest acceptable mid-term safety of chimney stenting. However, these retrospective data in a relatively small set of patients should be interpreted with caution. Therefore, we discourage the use of chimney stenting as a first-line strategy in younger patients or in those with advanced coronary artery disease, and chimney stenting should only be effectuated as a bailout option for impending or established CAO.
Future Perspective
Few registries have retrospectively collected data on this subject, demonstrating that chimney stenting is an effective bailout strategy for the acute management of CAO during TAVI, but long-term outcomes remain unclear. A prospective international registry collecting data on patients who are at risk of CAO or have developed CAO during TAVI is in development. Such data are needed to improve current risk models and enable the development of new tools to better predict the occurrence of CAO. Further experience with new techniques, such as BASILICA or THV design innovation, will also be required to mitigate the risk of coronary occlusion and the requirement for chimney stenting.
Conclusion
Acute CAO during TAVI is a rare but life-threatening complication. In most cases, risk factors for CAO can be identified. In patients at risk of CAO, upfront coronary protection with a coronary guide wire and the use of a premounted stent are suggested. Chimney stenting is performed as a bailout treatment for the restoration of coronary flow in case of impending or established CAO. The long-term performance of chimney stenting remains unclear.