Bicuspid aortic valve is the most common congenital cardiac malformation, affecting 1.3 % of the population, and responsible for a significant proportion of aortic valve replacement in adults.1-3 Clinical presentation varies from severe valve disease in infancy to asymptomatic valve in old age, but symptoms typically develop in adulthood. Surgical aortic valve replacement is generally required at an earlier age than surgeries for degenerative tricuspid aortic disease.3 However, in some patients, bicuspid aortic valve disease progresses in their 80s, but surgical risk is often extremely high due to age and multiple comorbidities.
Transcatheter aortic valve implantation (TAVI) is a safe and effective therapy for intermediate- and high-risk surgical patients with symptomatic severe aortic stenosis (AS), and more than 250,000 patients have been treated with this technology.4-9 Landmark randomised trials showed TAVI is a safe and effective standard treatment in patients classed as inoperable and is a reasonable option in patients at increased surgical risk, but these trials excluded congenital bicuspid AS due to its unique morphological features.4,5,8-10 Nevertheless, growing experience, accumulated knowledge, and advances in transcatheter heart valves have led to an expanded use of TAVI in lower risk surgical populations and in other pathologies such as bicuspid AS.
Initally, the use of TAVI in bicuspid AS was limited to small series.11–15 Previous registries showed that the proportion of patients with bicuspid AS treated with TAVI may reach 2–6 %.16,17 Considering the worldwide shift of treating younger and lower surgical-risk patients with TAVI and higher prevalence of bicuspid AS in younger populations, the clinical outcomes of TAVI in bicuspid AS warrants special attention.9,18,19 Furthermore, although diagnosis of bicuspid AS has been traditionally based on pathological findings, integration of multidetector computed tomography (MDCT) into pre-procedural assessment for TAVI is providing new insight. This review article described outcomes of TAVI in bicuspid AS and advances in diagnosis of bicuspid AS.
Outcomes of TAVI in Bicuspid Aortic Stenosis
The first use of TAVI in bicuspid AS was reported by Wijesinghe et al.20 Sapien valves (Edwards Lifesciences) were implanted successfully in 11 patients, resulting in significant haemodynamic improvement, a reduction of mean aortic valve gradient from 41 mmHg to 13 mmHg and an increase of aortic valve area from 0.7 cm2 to 1.5 cm2. However, 2 patients (18.2 %) had moderate paravalvular leak.
Hayashida et al. reported the outcomes of 21 patients with bicuspid AS and compared the outcomes between bicuspid and tricuspid AS.21 Despite limited sample size, they reported comparable outcomes of TAVI between bicuspid AS and tricuspid AS. CoreValve (Medtronic) was used more frequently in the bicuspid AS group (47.6 % versus 16.3 %; p=0.002). There was no significant difference in aortic regurgitation ≥ grade 2 (19.0 % versus 14.9 %; p=0.54), 30-day mortality (4.8 % versus 8.2 %; p=1.00) and device success rate (100 % versus 92.8 %; p=0.37).
Mylotte et al. showed the feasibility of TAVI in a large cohort (n=139) of bicuspid aortic valve stenosis using the first-generation balloon-expandable valves (Sapien [Edwards]; n=48) or self-expanding valves (CoreValve [Medtronic]; n=91).11 Mean age was 78.0 ± 8.9, and 56.1 % of patients were male with a mean Society of Thoracic Surgeons (STS) score of 4.9 ± 3.4, indicating intermediate surgical risk. The type of bicuspid aortic valve was available in 120 patients; type 0 in 26.7 %, type 1 in 68.3 %, and type 2 in 5.0 %. Paravalvular leak ≥ grade 2 occurred in 28.4 % of patients (19.6 % Sapien versus 32.2 % CoreValve; p=0.11). A new pacemaker was implanted in 23.2 % of patients (16.7 % Sapien versus 26.7 % CoreValve; p=0.21). One-year mortality was 17.5 %, without significant difference between the valves (20.8 % Sapien versus 12.5 % CoreValve; p=0.12). As in patients with degenerated tricuspid AS, the use of pre-procedural MDCT for device sizing was associated with a lower rate of significant paravalvular leak (OR 0.19; 95 % CI [0.08–0.45]; p<0.001). Major vascular complication was identified as a significant predictor of 1-year all-cause mortality (OR 5.66; 95 % CI [1.21–26.42]; p=0.03), which was consistently observed in tricuspid AS population. Interestingly, paravalvular leak ≥ grade 2 was not associated with 1-year all-cause mortality (OR 1.55; 95 % CI [0.56–4.32]; p=0.40). This work showed the safety and efficacy of TAVI in selected patients with bicuspid AS, but the high rate of significant paravalvular leak with these devices was alarming.
Newer devices designed to overcome these limitations showed superior procedural outcomes in tricuspid AS compared to the early devices. Accordingly, these devices were used in bicuspid AS, with the expectation that they would overcome the procedural challenges resulting from the unique anatomic features of bicuspid AS.
Perlman et al. reported improved outcomes using a new-generation balloon-expandable valve (Sapien 3, Edwards Lifesciences).12 Among 51 patients with bicuspid AS treated with the Sapien 3, none had second valve implantation or paravalvular leak ≥ moderate. New permanent pacemakers were implanted in 23.5 % of patients, a relatively higher rate than in tricuspid AS. The low implantation of the transcatheter valves was associated with more frequent new permanent pacemaker implantation, which was also observed in tricuspid AS patients. Less oversized devices (area oversizing < 10 %) tended to have more frequent paravalvular leak >mild compared to those with more oversized devices (area oversizing ≥10 %; 48 % versus 26.9 %; p=0.10). Given that no moderate or greater paravalvular leak was observed in this study, and the fact that using more oversizing devices may carry the risk of annulus rupture or aortic injury, the selection of less oversized devices may be a reasonable option. Post-procedural echocardiography at 30 days showed a slightly smaller aortic valve area in patients who received the less oversized devices (1.56 ± 0.27 cm2 versus 1.78 ± 0.33 cm2; p=0.01), which could be a potential cause of future deterioration in valve function. Future studies are awaited to clarify the association of valve haemodynamics and selection of device size.
The Bicuspid AS TAVI registry included 301 patients with bicuspid AS from 20 centres in Europe, North America and Asia-Pacific.13 The mean age was 77.0 ± 9.2 with mean STS score of 4.7 ± 5.2 % (intermediate surgical risk). Early-generation devices were used in 199 patients (Sapien XT: n=87; CoreValve: n=112) and new-generation devices in 102 patients (Sapien 3: n=91; Lotus [Boston Scientific]: n=11). Pre-procedural MDCT was performed in 86.0 % of patients with a significantly higher rate in the new-generation device group (100.0 % versus 78.9 %; p<0.001), reflecting preprocedural MDCT assessment as a gold standard. Transfemoral access was used more frequently in the new-generation device group (95.1 % versus 78.4 %; p<0.001). Procedure-related death, conversion to surgery and coronary obstruction occurred in 1.3 %, 2.9 %, and 1.0 % of patients overall, with no significant differences between the groups. New-generation devices were associated with less frequent second valve implantation (1.0 % versus 6.5 %; p=0.04) and moderate or greater paravalvular leak (0.0 % versus 8.5 %; p=0.002), which resulted in higher device success rate (92.2 % versus 80.9 %; p=0.01) (Figure 1). Annulus rupture occurred more frequently with the Sapien XT than the CoreValve (4.6 % versus 0.0 %; p=0.04). Second valve implantation was more frequent with the CoreValve compared to the Sapien XT (10.7 % versus 1.1 %; p=0.007) and the Sapien 3 (10.7 % versus 1.1 %; p=0.005).
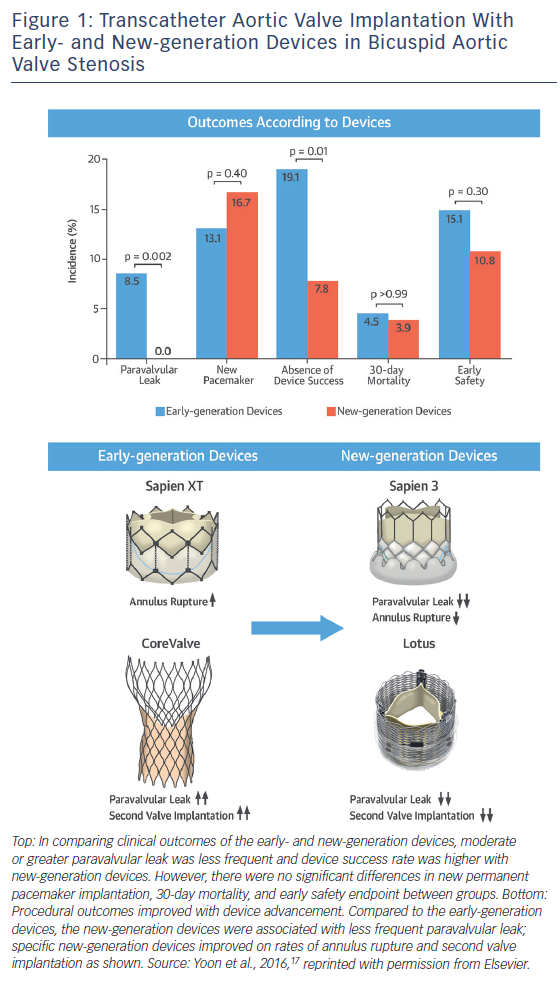
There were no significant differences in new permanent pacemaker insertions between devices, although there was a trend towards a higher rate with the Sapien 3 compared to the Sapien XT (17.6 % versus 9.2 %; p=0.10). Device success was higher with the Sapien 3 compared to the Sapien XT (94.5 % versus 85.1 %; p=0.04) and the CoreValve (94.5 % versus 77.7 %; p=0.001). There were no significant differences in major 30-day endpoints between the early- and new-generation device groups (all-cause 30-day mortality: 4.5 % versus 3.9 %; p>0.99; stroke: 2.5 % versus 2.0 %; p>0.99; life-threatening bleeding: 3.5 % versus 2.9 %; p>0.99; major vascular complications: 4.5 % versus 2.9 %; p=0.76; stage 2 or 3 acute kidney injury: 15.1 % versus 10.8 %; p=0.30). The higher rate of annulus rupture with the Sapien XT may be attributable to the nature of balloon-expandable heart valves and the need for selection of oversized valves to prevent paravalvular leak and device embolisation. The Sapien 3 has an external skirt, so the selection of extremely oversized valves may be no longer required.
For patients with challenging anatomy, such as heavily calcified leaflet and raphe, less oversized balloon-expandable devices can be selected. The early generation self-expanding CoreValve was associated with more frequent paravalvular leak. Due to the complex anatomy of bicuspid aortic valve, annulus measurement with MDCT may not be accurate in patients with bicuspid AS, and supra-annular structure may have a potential role in anchoring the transcatheter valves, particularly self-expanding valves. Liu et al. performed sequential balloon aortic valvuloplasty before TAVI with self-expanding valves.22 In 12 patients, 11 (91.7 %) received smaller devices according to the sequential balloon sizing compared to the annulus measurement with MDCT without any significant complications, including moderate or severe paravalvular leak. This suggests the potential role of balloon sizing, but the subsequent risk of adverse events such as aortic injury or stroke should be considered.
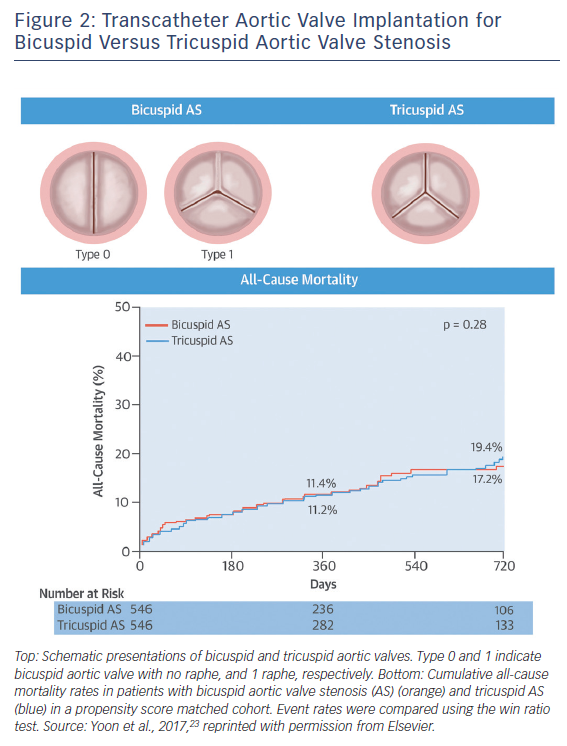
In terms of intermediate- and long-term survival data in bicuspid AS, studies were limited by the clear differences in age and comorbidities favouring the bicuspid AS population compared with tricuspid AS population. Given that patients with bicuspid AS are younger and have fewer coexisting comorbidities compared with tricuspid AS, there is a potential risk that clinical outcomes of TAVI for bicuspid AS could differ from those for tricuspid AS with equivalent surgical risk. A recent study compared the outcomes of TAVI between the bicuspid and tricuspid AS populations using propensity-score matching.23 A total of 576 patients with bicuspid AS from 33 centres and 5,900 patients with tricuspid AS were included in this analysis, and 546 pairs of patients with bicuspid and tricuspid AS were created. In the unadjusted cohort, patients with bicuspid AS were younger and more likely to be male, whereas patients with tricuspid AS were more likely to have multiple comorbidities. Accordingly, patients with bicuspid AS had a lower Logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) (14.8 ± 12.3 % versus 16.7 % versus 11.8 %; p=0.003) and STS score (5.0 ± 5.1 % versus 6.5 ± 8.8 %; p<0.001). After performing propensity score matching, both groups were well matched, with no significant differences in baseline characteristics.
In the propensity score-matched cohort, patients with bicuspid AS had more frequent conversion to surgery compared to those with tricuspid AS (2.0 % versus 0.2 %; OR 11.00; 95 % CI [1.42–85.20]; p=0.006) and second valve implantation (4.8 % versus 1.5 %; OR 3.71; 95 % CI [1.61–8.56]; p=0.002), and moderate or severe paravalvular leak (10.4 % versus 6.8 %; OR 1.61; 95 % CI [1.04–2.48]; p=0.04), leading to lower device success rate (85.3 % versus 91.4 %; OR 0.54; 95 % CI [0.37–0.80]). When using the early-generation devices, the more frequent adverse events in bicuspid AS compared to tricuspid AS were consistently observed (conversion to surgery: 2.5 % versus 0.3 %; p=0.02; second valve implantation: 7.2 % versus 2.2 %; p=0.003; moderate or severe paravalvular leak: 15.9 % versus 10.3 %; p=0.03; device success, 78.4 % versus. 86.9 %; p=0.005). In contrast, there were no significant differences between groups in procedural complications with the new-generation devices (conversion to surgery: 1.3 % versus 0.0 %; p=0.25; second valve implantation: 1.3 % versus 0.4 %; p=0.62; moderate or severe paravalvular leak: 2.7 % versus 1.8 %; p=0.53; device success: 95.1 % versus 97.8 %; p=0.13; new pacemaker implantation: 16.4 % versus 17.8 %; p=0.69).
In terms of midterm mortality, the cumulative all-cause mortality rates at 2-year follow-up were comparable between the bicuspid and tricuspid AS groups (17.2 % versus 19.4 %; p=0.28) (Figure 2). Furthermore, there were no significant differences in 1-year all-cause mortality rates between the groups stratified according to the early- and new-generation devices (early-generation devices: 14.5 % versus 13.7 %; p=0.80; new-generation devices: 4.5 % versus 7.4 %; p=0.64). Compared to tricuspid AS, TAVI in bicuspid AS was associated with lower device success rate due to challenging anatomy, but 1-year mortality rates were similar. Procedural differences were observed in patients treated with the early-generation devices, whereas no differences were observed with the new-generation devices. Nevertheless, the unique anatomic features of bicuspid AS – including eccentric distribution of calcification and calcified raphe – may hinder the expansion of transcatheter heart valves. This raises the concerns about long-term valve durability in bicuspid AS population. Given higher prevalence of bicuspid AS in younger population, the expanding use of TAVI to younger and less comorbid populations warrants long-term durability data in the bicuspid AS population.
CT for Diagnosis of Bicuspid Aortic Valve
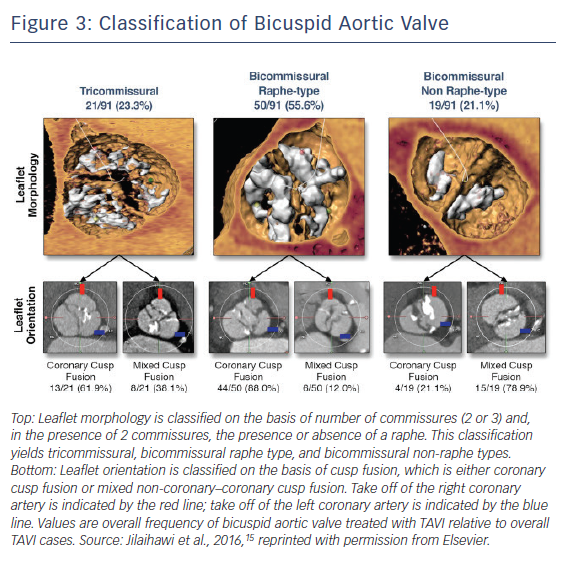
Pre-procedural MDCT assessment of aortic valve has become standardised in the TAVI era, and pre-procedural diagnosis of bicuspid AS with MDCT has gained considerable attention. With accumulation of experience in MDCT assessments of aortic valves, the great variety of bicuspid AS morphology has been observed, leading Jilaihawi et al. to propose a more simplified, TAVI-directed classification of bicuspid aortic valve.15 Bicuspid aortic valves were classified according to the numbers of raphe and commissures with high-resolution MDCT images (Figure 3). This classification specified three bicuspid aortic valve morphologies as tricommissural (one commissure completely fused between two cusps, often referred to as functional or acquired bicuspid aortic valve); bicommissural raphe type (two cusps are fused by a fibrous or calcified ridge of various heights which does not reach the height of the commissure); and bicommissural non-raphe type (two cusps completely fused from their basal origin with no visible seam).
Among 21 patients with tricommissural bicuspid aortic valves and 70 patients with bicommissural bicuspid aortic valves, there were no significant differences in annulus dimensions (mean maximum diameter: 27.6 ± 2.9 mm versus 27.6 ± 3.3 mm; p=0.96; mean minimum diameter: 22.2 ± 2.2 mm versus 22.3 ± 2.8 mm; p=0.82; annulus area: 472.5 mm2 versus 486.4 mm2; p=0.57; annulus perimeter: 78.2 mm versus 79.4 mm; p=0.52) except larger intercommissural distance and ascending aorta in bicommissural group (intercommissural distance: 28.7 mm versus 24.3 mm; p<0.001; ascending aorta: 40.9 mm Sapien 3 36.0 mm; p<0.001). When comparing bicommissural non-raphe type (n=19) and bicommissural raphe type (n=50), bicommissural raphe type had larger annulus dimensions (annulus area 505.0 mm2 versus 434.4 mm2; p=0.015; annulus perimeter 80.9 mm versus 75.0 mm; p=0.016), but the diameter of ascending aorta was similar between the two groups (40.5 mm versus 42.5 mm; p=0.25). Interestingly, intercommissural distance was associated with paravalvular leak in bicommissural but not tricommissural bicuspid AS. Further studies are needed to evaluate the impact of this parameter in treating patients with bicuspid AS. This classification may clarify the difference between functional/acquired bicuspid aortic valves and bicuspid aortic valves with one raphe (Sievers’ type 1) on the basis of MDCT images.
Based on this classification, Kim et al. performed a systematic review pre-procedural MDCT images in a large contemporary TAVI cohorts to accurately determine the prevalence of bicuspid aortic valve and to compare clinical outcomes with patients with tricuspid aortic valve stenosis.24 MDCT images of 1,996 patients were retrospectively reviewed by two experienced readers for the presence of bicuspid AS. After exclusion of undetermined cases (n=20) or tricuspid with acquired fusion (n=60), bicuspid AS was confirmed in 144 patients (7.3 %). After adjustment for baseline differences with propensity matching, comparing to patients with tricuspid AS, those with bicuspid AS had more frequent paravalvular regurgitation ≥ grade 2 (11.1 % versus 2.8 %; p=0.005), and tended to have more frequent aortic root injury (4.2 % versus 0.7 %; p=0.056), whereas all-cause mortality rates were similar at 30 days (4.9 % versus 3.5 %; p=0.55) and 1 year (18.2 % versus 19.8 %; p=0.74). This study provided a comprehensive overview of the various spectra of bicuspid aortic valve morphologies. Given that bicuspid AS is associated with procedural challenges, the proper identification and classification of bicuspid AS phenotypes is essential.
Conclusion
TAVI has led to changes in the diagnosis and classification of bicuspid aortic valve. Due to unfavourable anatomic features of bicuspid aortic valves, the outcomes of TAVI in bicuspid AS were suboptimal, particularly when using early generation devices. However, new generation devices improved the outcomes of TAVI in bicuspid AS comparable to those of tricuspid AS.