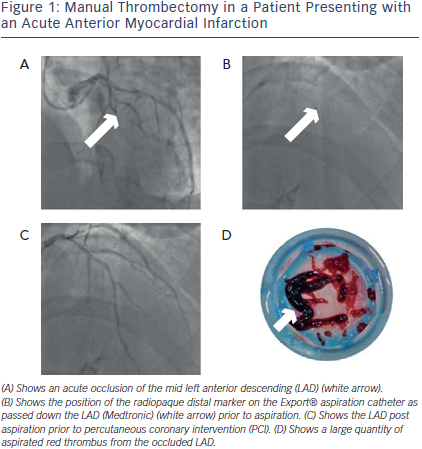
Primary percutaneous coronary intervention (PPCI) is the optimal treatment strategy for restoring coronary blood flow in the infarct related artery (IRA) and salvaging myocardium in patients with ST elevation myocardial infarction (STEMI).1,2 Despite this, PPCI fails to restore optimal myocardial perfusion in up to 40 % of patients despite restoring epicardial artery patency as evidenced by failure of ST-segment resolution (STR) and poor myocardial blush grade (MBG).3 This failure of microvascular perfusion is associated with larger infarct size, left ventricular dysfunction and decreased survival in the long term.4– 6
Even though different mechanisms underlie microvascular injury after PPCI, such as generation of reactive oxygen species, cardiomyocyte calcium overload, vasoconstriction, inflammation and cellular and interstitial oedema, distal embolisation appears to play a pivotal role.7,8 A number of studies have demonstrated that PCI can result in an embolisation rate of up to 15 %.8,9 Embolisation of large particles, such as plaque debris, can result in occlusion of pre-arterioles and small side branches, whereas microembolisation can result in occlusion of arterioles and capillaries that can impair perfusion of the myocardium at a microscopic level. 10,11 A high thrombus burden is associated with an increased incidence of distal embolisation and itself has been associated with higher frequency of adverse outcomes (major adverse cardiac events [MACEs] and mortality).12
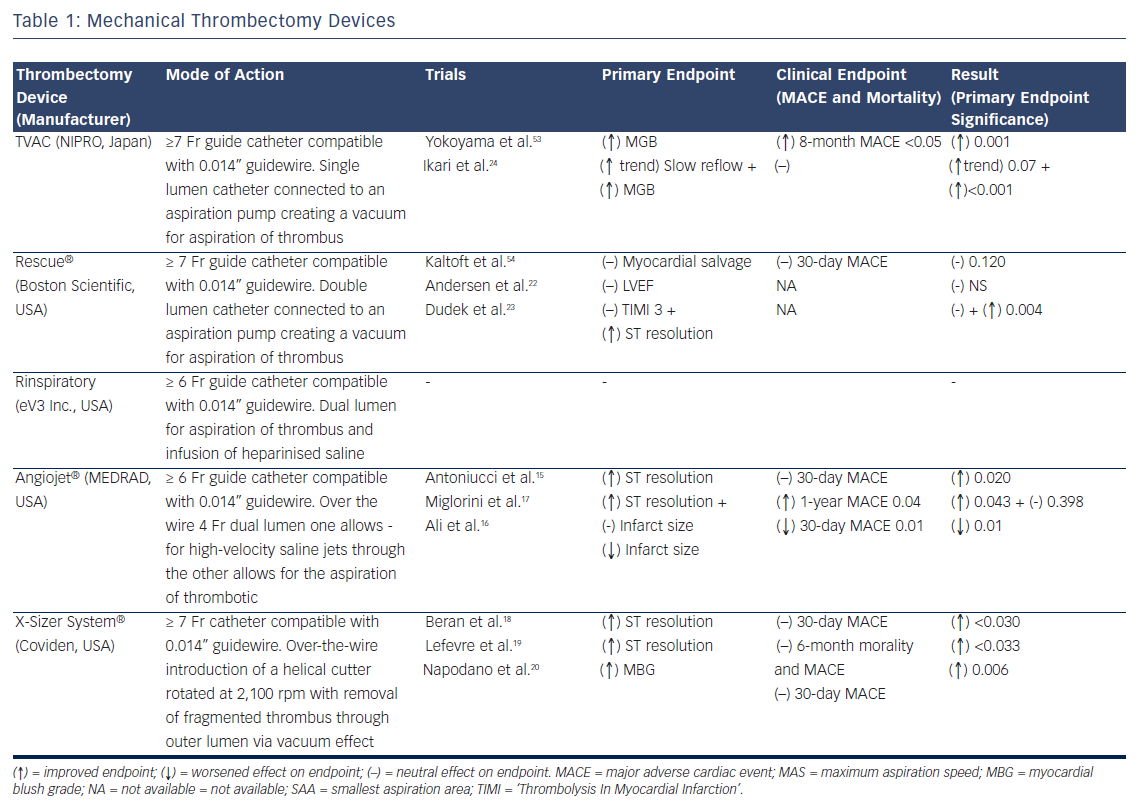
Strategies, such as thrombectomy devices, to help reduce thrombus burden and prevent distal embolisation have been proposed to increase microvascular flow and improve outcomes.13,14 As this embolisation occurs predominantly at the time of the initial balloon or stent inflation, using a thrombectomy device to reduce thrombus burden before inflation may decrease the likelihood of distal embolisation (see Figure 1). This has led to the development of a number of devices to prevent this problem occurring.11 Currently, a variety of commercially available devices are available with different mechanisms that enable them to either fragment or aspirate the thrombus (see Table 1 and 2). Thrombectomy devices are broadly divided into two groups depending on whether they are motorised (mechanical thrombectomy) or not (manual thrombectomy).
Mechanical Thrombectomy
Mechanical thrombectomy devices have several mechanisms: some actively fragment thrombotic material before aspiration such as the AngioJet® (MEDRAD, USA), X-Sizer® (Coviden, USA) and Rinspirator™ System (eV3 Inc., USA) whereas others carry out mechanical aspiration only e.g. the TransVascular Aspiration Catheter® (Nipro, Japan) and Rescue™ (Boston Scientific, USA) devices.
The AngioJet system has been assessed in three randomised trials with conflicting results. Initially a single-centre study of 100 patients showed a reduction in infarct size and improved STR compared with PPCI alone;15 however, the larger AngioJet Rheolytic Thrombectomy In Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction (AiMI) trial (480 patients) found no advantage of the AngioJet system in terms of ‘Thrombolysis In Myocardial Infarction’ (TIMI) 3 flow, MBG or STR compared with PPCI alone.16 In fact, final infarct size and MACE rates at 30 days were higher in the thrombectomy group. Subsequently, the Comparison of AngioJet Rheloyic Thrombectomy Before Direct Infarct Artery Stenting With Direct Stenting Alone in Patients with Acute Myocardial Infarction (JETSTENT) trial17 recruited 501 patients with STEMI who were randomised to mechanical thrombectomy before direct stenting or to direct stenting alone. Unlike the AiMI study, this study required patients to have angiographically visible thrombus before they were recruited into the study. The study demonstrated no significant differences between the two groups in STR, TIMI 3 flow, TIMI blush grade 3 or infarct size (as assessed by nuclear scanning). However, the mechanical thrombectomy group had reduced MACE at 6 months and improved 1-year event-free survival rates.17 However, the improved clinical outcomes should be interpreted with caution as with no difference in infarct size or myocardial perfusion between the groups, the mechanism behind the significant clinical benefit is unclear.
In the X-Sizer in AMI for Negligible Embolization and Optimal ST Resolution (X AMINE ST) trial, the X-Sizer System® was investigated in 201 patients with STEMI undergoing PPCI. Although the study demonstrated improved STR at 60 minutes post-PCI and demonstrated reduced distal embolisation of debris and lower no reflow rates, it did not demonstrate any significant clinical benefit at 1 and 6 months.18–20 Furthermore, the X-Sizer has been associated with increased rates of coronary artery perforation in other studies.21
In terms of mechanical thrombectomy devices that aspirate thrombus without fragmentation, only the TransVascular Aspiration Catheter has demonstrated positive results with the Rescue® system not associated with any significant improvement in infarct size, MBG or left ventricular ejection fraction in randomised trials.22,23 The VAcuuM asPIration thrombus REmoval (VAMPIRE) study24 was a randomised trial comparing the TransVascular Aspiration Catheter versus PCI alone that showed a small improvement in TIMI flow and MBG. Similar MACE rates were seen at 30 days between the groups; however, a significant reduction in MACE at 8 months in the thrombectomy group was seen, mainly driven by lower revascularisation rates in this group. Importantly no difference in mortality was seen.
Table 1 lists the manual thrombectomy devices currently available as well as the randomised clinical trials investigating their use in STEMI.
Manual Thrombectomy
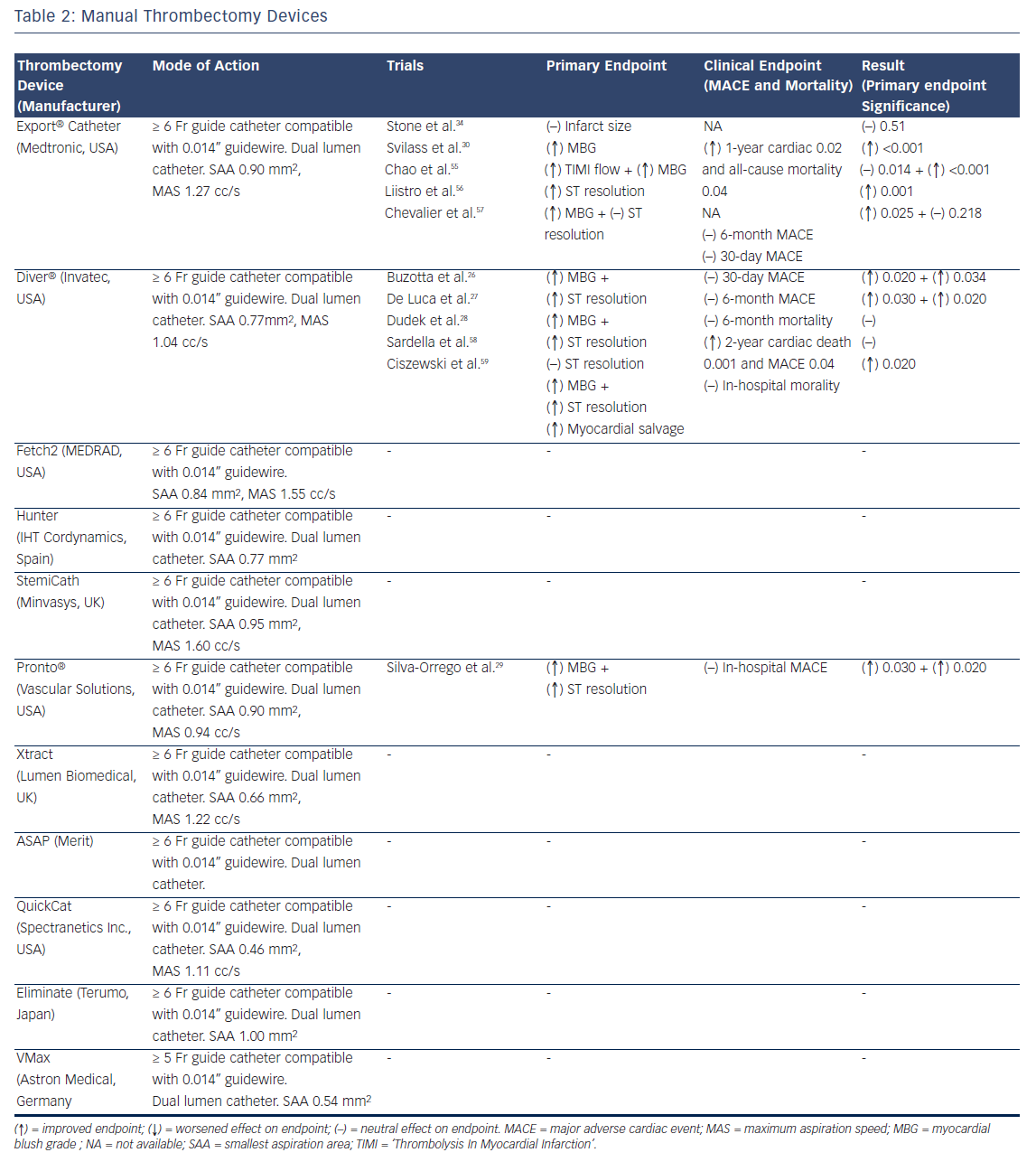
Manual thrombectomy devices are simpler to use in comparison to mechanical thrombectomy devices. However, due to their mechanism, manual devices cannot extract large amounts of thrombus compared with mechanical thrombectomy devices, which can result in distal embolisation of thrombotic material.25 Manual thrombectomy devices that are currently in clinical use include the Export® Catheter (Medtronic, USA), Hunter (IHT Cordynamics, Spain), Diver® (Invatec, Italy), QuickCat (Spectranetics Inc., USA), Pronto® (Vascular Solutions, USA) and Eliminate (Terumo) among others. Although these devices are similar, they differ in terms of aspiration, lumen size and configuration and there are some differences in the way the thrombus is extracted.
Clinical Trial Data
Manual thrombectomy in PPCI for STEMI has been assessed in a number of clinical trials. The first randomised trial that tested a manual aspiration device was the Randomised Evaluation of the Effect of Mechanical Reduction of Distal Embolisation by Thrombus Aspiration in Primary and Rescue Angioplasty (REMEDIA) study (Diver®). This study randomised 99 patients to PCI with manual aspiration or PCI only.26
This study demonstrated that manual aspiration was associated with significantly better STR and MBG as well as reduced no reflow and distal embolisation. Furthermore, a subgroup analysis demonstrated that thrombus aspiration appeared to be more beneficial in patients with occluded arteries and a higher thrombus burden.26 However, no associated clinical benefit was seen, as the study was underpowered. De Luca et al. found that using manual thrombectomy in patients with anterior STEMI (n=76), demonstrated better post-procedural MBG and better STR at 90 minutes.27 However, again, these findings were not translated into improved clinical outcomes because the study was underpowered. Similar findings were seen in larger studies: Polish-Italian-Hungarian RAndomized ThrombEctomy (PIHRATE) trial (196 patients) and Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction (DEAR-MI) (Pronto catheter, 148 patients) with again no improved clinical outcomes.28,29 However, the translation of improved procedural outcomes into better clinical outcomes was achieved with the publication of the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction (TAPAS) trial.
TAPAS
The majority of randomised trials have shown that manual thrombectomy is associated with improved MBG, STR and TIMI flow. However, until TAPAS, most of these studies were not powered to detect a clinical benefit. The TAPAS study was a single-centre randomised trial that randomised 1,071 patients with STEMI in a 1:1 fashion to manual aspiration using the Export catheter or PCI alone.30 Patients in the thrombectomy arm had higher MBG, improved STR and fewer pathological Q-waves. Significantly, these beneficial effects on reperfusion resulted in fewer clinical events at both 30 days (reduced mortality and re-infarction) and a significant reduction in mortality at 1 year.31
After TAPAS, other trials have corroborated the benefits of manual thrombectomy. In the Thrombectomy With export Catheter in Infarct- Related Artery During Primary Percutaneous Coronary Intervention (EXPIRA) trial, 175 patients were recruited with MBG, STR and microvascular obstruction (MVO) as primary endpoints. This trial was the first study to assess thrombectomy use with MVO as a primary endpoint.32 The study demonstrated that manual thrombectomy significantly improved both MBG and STR, reduced MVO and final infarct size (as assessed by cardiac magnetic resonance [CMR]) and was associated with reduced cardiac mortality and MACE at 24-month follow-up.32
However, unlike TAPAS and other single- centre trials, multi-centre studies have mostly been negative. The INFUSE-AMI trial (which was a Factorial, Randomized, Multicentre, Single-Blind Evaluation of Intracoronary Abciximab Infusion and Aspiration Thrombectomy in Patients Undergoing Percutaneous Coronary Intervention for Anterior ST-Segment Elevation Myocardial Infarction) recruited 452 patients presenting early with large anterior STEMI undergoing PPCI. They were randomised in a 2 x 2 factorial design to bolus intracoronary abciximab versus no abciximab and to manual aspiration thrombectomy versus no aspiration.33 Here, manual thrombus aspiration was not effective in reducing infarct size as assessed by CMR or MACE at 30 days.34 Importantly, and most significantly, the recently published Thrombus Aspiration in Myocardial Infarction (TASTE) study is the largest study performed to date and was also negative for clinical improvement. This was a multicentre, prospective, randomised controlled trial (RCT), which randomised 7,244 patients with STEMI undergoing PPCI to manual thrombus aspiration versus PCI alone, with the primary endpoint of 30-day mortality. Manual devices used included Eliminate, Export and Pronto. Although there was a trend towards a reduction in rates of re-infarction at 30 days in the thrombectomy group, there was no significant difference in 30-day mortality between the two groups.35 In addition, there were no significant differences in rates of stroke, heart failure, left ventricular function or stent thrombosis between the two groups.35
Table 2 lists the manual thrombectomy devices currently available as well as the randomised clinical trials investigating their use in STEMI.
Meta-analyses
A number of randomised trials have investigated mechanical and manual thrombectomy; however, until recently these have been mainly small studies with short follow-up periods. Hence, there have been many meta-analyses that have investigated the use of thrombectomy in the setting of PPCI, which have produced conflicting results.
A meta-analysis by Kumbhani et al., which included 3,936 patients comparing manual/mechanical thrombectomy versus PCI alone from 18 trials showed that manual aspiration was associated with a benefit in reducing MACE, including mortality at 6 to 12 months compared with PCI alone.36 This was supported by the Long-Term Clinical Efficacy of Thrombectomy Devices in Acute ST-Elevation Myocardial Infarction (ATTEMPT) meta-analysis, which pooled analyses on 2,686 individual patient’s data from 11 randomised trials.37 ATTEMPT demonstrated that at a median of 1-year follow-up, all-cause mortality, MACE, death and myocardial infarction were significantly lower in the thrombectomy group.37 However, interestingly, survival benefit was confined to patients treated with manual thrombectomy alone with an estimated 34 patients needed to be treated to prevent one death at 1 year.37 Further metaanalyses by Costopoulos et al., which combined 10 randomised trials, finding that manual thrombectomy was associated with better MBG, STR and TIMI 3 flow rates as well as reduced mortality (43 %; p=0.04), which contrasted to mechanical thrombectomy, where no benefit was seen,38 and by Bavry et al., which showed a significant increase in mortality in patients treated with mechanical thrombectomy compared with PCI alone (5.3 % versus 2.8 %, respectively).39
However not all meta-analyses have been positive. A Bayesian metaanalysis by Mongeon et al., which included 21 trials totalling 4,299 patients (16 trials that used manual aspiration thrombectomy device), thrombectomy was shown to result in more STR and TIMI 3 flow.40 However, there were no significant reductions in death, recurrent MI or stroke at 30 days post procedure. The results were similar when analysis was confined to manual thrombectomy.40 It was felt that the overall number of endpoints were low and follow-up periods were short, which may explain why no differences were seen in clinical endpoints.41 Despite this, another more recent meta-analysis by Tamhane et al., consisting of 3,904 patients, also did not detect a difference in 30-day mortality42 despite a trend towards improved survival rates with the use of manual thrombectomy (odds ratio, 0.57;p=0.05). De Luca et al., conducted the most recent meta-analysis, which included 4,514 patients from 21 RCTs undergoing manual or mechanical thrombectomy, showed that thrombectomy did not reduce 30-day mortality, or reinfarction. Manual but not mechanical thrombectomy was shown to significantly improve post-procedural TIMI 3 flow. Importantly this meta-analysis raised the question that thrombus aspiration may not be a risk-free procedure. Systemic embolisation can occur, and in this meta-analysis, thrombus aspiration was associated with a trend towards an increased rate of stroke (p=0.06)43 – this was noted with both types of thrombectomy device.
Manual versus Mechanical
Evidence suggests that manual thrombectomy provides the most benefit in PPCI as demonstrated by several meta-analyses.36,37,39,43 On the other hand, mechanical thrombectomy may provide limited benefit and possibly cause harm.39 There could be a number of explanations for the discouraging results seen with mechanical thrombectomy. First, mechanical thrombectomy devices are often complex to setup and operate compared with manual thrombectomy devices resulting in a steeper learning curve. Staff familiarity with the use of these devices is probably limited, especially as most PPCIs occur out of hours44 when staff levels are reduced. Second, mechanical thrombectomy decides are larger and have a longer setup time that, in turn, results in a longer procedure time. This has been demonstrated where, in contrast to manual thrombectomy studies,33,37,45,46 all mechanical thrombectomy studies19,31,40,47 have longer procedural times compared with PPCI without thrombectomy use.
Current Guidelines
Both the current European Society of Cardiology (ESC) and American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend coronary artery thrombus aspiration as adjunctive therapy during primary PCI for STEMI.48,49 These recommendations are based partly on TAPAS30 together with several meta-analyses,37,39 which provided the necessary evidence to endorse thrombus aspiration as a class IIa recommendation with the level of evidence B in the ACC/AHA guideline48 and with level of evidence A in the ESC guidelines.49 Whether the recent data should change this is much debated. Thrombus aspiration clearly has a role to play but perhaps the routine use is not the answer. Instead, the use of thrombectomy should probably be limited to cases of poor pre-procedural reperfusion or in cases where there is evidence of large intracoronary thrombus burden.
The Future
Due to the uncertainty of the use of thrombectomy in PPCI for STEMI, a number of large multi-centre clinical trials are currently taking place, which will hopefully provide a more definite answer to whether the use of thrombectomy is associated with a clinical benefit in this setting. Further evidence regarding routine use should be provided by the large multi-centre trial to date investigating the role of manual aspiration thrombectomy using the Export catheter (A Trial of Routine Aspiration Thrombectomy With PCI Versus PCI Alone in Patient with STEMI Undergoing Primary PCI [TOTAL]) is aiming to recruit 4,000 patients. Primary composite endpoints are cardiovascular death, recurrent MI, cardiogenic shock, or new or worsening New York Heart Association (NYHA) Class IV heart failure up to 180 days.50
A direct comparison between manual and mechanical will be provided by the Comparison of Manual Aspiration With Rheolytic Thrombectomy in Patients Undergoing Primary PCI (SMART-PCI) trial. This is a singlecentre study that is directly comparing the role of mechanical versus manual thrombectomy in PPCI.47,51 The primary endpoint is residual thrombus burden assessed as number of coronary quadrants containing thrombus by optical coherence tomography (OCT) after thrombectomy and before infarct artery stenting. Their preliminary results suggest that mechanical thrombectomy has better STR, TIMI 3 flow and TIMI grade 3 blush compared with manual thrombectomy.51 However, this study is not powered to investigate any long-term clinical benefits of thrombectomy.
Finally, a large ongoing trial in Korea including 27 centres is comparing PPCI using thrombectomy with PPCI alone. They are aiming to recruit 1,400 patients in total with a primary endpoint of cardiac death and MI at 12 months after their procedure.52
Although one cannot be certain whether these trials will provide a definitive answer regarding the use of thrombectomy in PPCI for STEMI, they will hopefully add clarity to the long-term benefits of their use.
Conclusion
Theoretically, thrombectomy appears to be a valuable approach to improve outcomes after PPCI and manual devices have demonstrated benefits on surrogate markers of reperfusion and clinical outcomes in many randomised trials and meta-analyses. However the largest RCT performed to date did not demonstrate an association between manual thrombectomy use and improved clinical outcomes. Mechanical thrombectomy, on the other hand, has failed to demonstrate any clinical benefit in the majority of studies performed including metaanalyses with some suggesting a harmful effect. Of concern, one recent meta-analysis highlighted potentially higher stroke rates with both forms of thrombectomy use although this was not seen in the TASTE study. Further large clinical trials in combination with registry data will gain confidence for mandating clinical change in practice in favour of thrombectomy should they prove positive. Until then, current evidence does not fully support routine use of thrombectomy.