Aortic dissection (AD) is one of the most challenging vascular diseases, with an in-patient mortality as high as 30 %1 and 30-day and 5-year fatality rates of just over 50 % and 60 %, respectively.2 The time course of AD is broadly spit into acute (<14 days), subacute (15–90 days), and chronic (>90 days) phases, and it is split clinically into complicated (i.e. the condition is associated with organ malperfusion syndrome, neurological symptoms and/or uncontrollable hypertension) and uncomplicated (when the patient is asymptomatic and haemodynamically stable) scenarios.3 The condition is most commonly classified according to the location of the primary entry tear (Stanford classification), with type A relating to the ascending aorta and type B the descending aorta. The dissection flap most commonly develops in the ascending aorta, occurring within 10 cm of the aortic valve in over 90 % of cases.4,5 Type A dissection has a mortality rate approaching 60 % if surgical intervention is not performed early on, yet – even with early intervention – mortality remains at 25 %, and neurological injury occurs in up to 14 % of cases, even in tertiary referral centers.4 The second most frequent site for the intimal tear is just distal to the left subclavian artery (LSA), which is a type B tear.3 In 5–10 % of dissections there is no clearly visible intimal tear, and the condition may be because of the rupture of the aortic vasa vasorum in the media layer, which is known as an intramural haematoma.3
Treatment of an AD is quite complex, as demonstrated by the high morbidity and mortality rates associated with surgical therapy. Endovascular technique is associated with a better outcome but presents some technical limitations secondary to the anatomical characteristics, especially in type A dissections. This is the reason treatment is currently evolving toward the new frontier of hybrid repair. Mitchell et al.5 addressed this concept in 2002, and it was subsequently applied by Criado et al.6 Five different landing zones (LZ0–4) have been defined in the aortic arch that can be used in different types of combined procedures.5,6 The concept of hybrid repair lies in repairing the ascending aorta (if involved) and extending the LZ to allow satisfactory sealing of the stentgraft according to the location of the intimal tear, combining open and endovascular procedures.7 Innovative and advanced surgical and endovascular techniques have recently been highlighted; this review aims to address the most recent techniques of hybrid repair in type A and B AD.
Hybrid Techniques
The classic surgical approach to AD requires cardiopulmonary bypass and deep hypothermic circulatory arrest. The aim is to identify and resect the primary intimal tear and re-approximate the intima and adventitia. Despite surgery, a residual dissection flap and a patent false lumen persist in the arch and descending thoracic aorta in 64–90 % of cases.3 A persisting patent false lumen can lead to distal malperfusion syndrome in the acute setting, with a risk of false lumen dilatation/ aneurysm formation and rupture over the long term.8
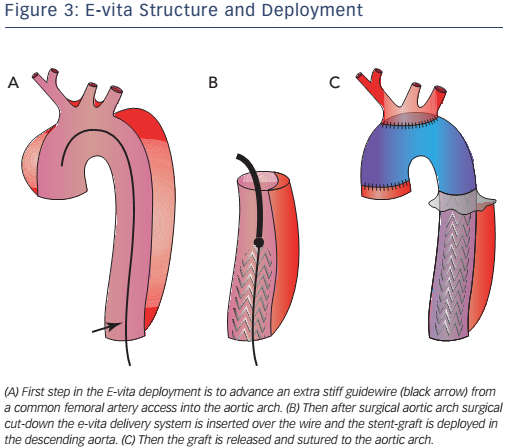
The overall mortality in type A dissection is almost three times higher in patients with malperfusion (43.7 %) than in those without (15 %).9 Hybrid techniques (see Table 1) are recommended in the latest guidelines for the treatment of type A AD with distal malperfusion.3 The following novel hybrid techniques overcome the limits of classic open surgery in the treatment of complex Stanford type A and B AD:
- elephant trunk (ET) techniques with or without thoracic endovascular aortic repair (TEVAR);
- revascularisation of the supra-aortic branches and TEVAR; and
- stent-graft repair without surgical supra-aortic vessel revascularisation.
Elephant Trunk Techniques
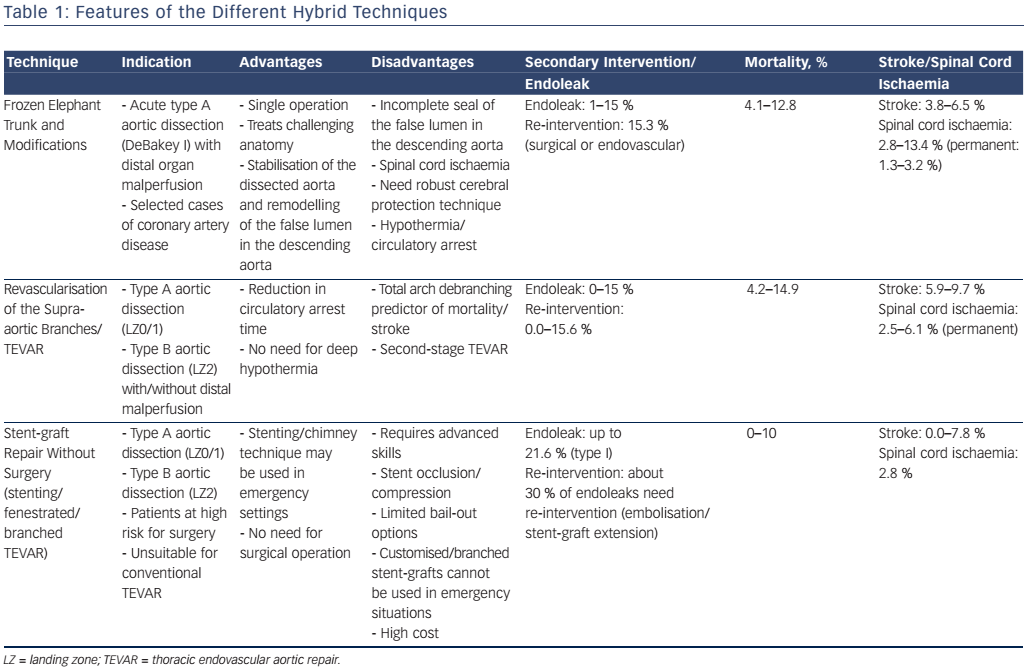
Generally ET open surgery techniques involve total arch replacement and distal anastomosis with various hypothermia techniques and periods of circulatory arrest. The original ET technique was introduced by Borst et al. in 1983 and consisted of a graft extending from the distal aortic arch into the proximal descending aorta, facilitating a second intervention with thoracotomy or via an endovascular approach10 (see Figure 1). This procedure is associated with a high risk of post-operative mortality and neurological events,10 therefore modifications have been applied such as a short ET, to reinforce the distal anastomosis, or a long ET (recommended length less than 10–12 cm), which improves aneurysm/false lumen thrombosis but has a high risk of paraplegia (8.1 %).10
With the advent of TEVAR, second-stage completion with an endovascular stent-graft is now available. This technique has the advantage of markedly shortening the time to second intervention, which can be performed safely within 1 month, and may be used in both thoracic aortic aneurysm and acute dissection. Reports show an in-hospital mortality of between 4.5 % and 11.1 %, a pooled mortality of 6.1 % and spinal cord ischaemia of 4.6 %.10,11 The main disadvantage appears to be late endoleak, which occurs in up to 7.4 % of cases.10,11
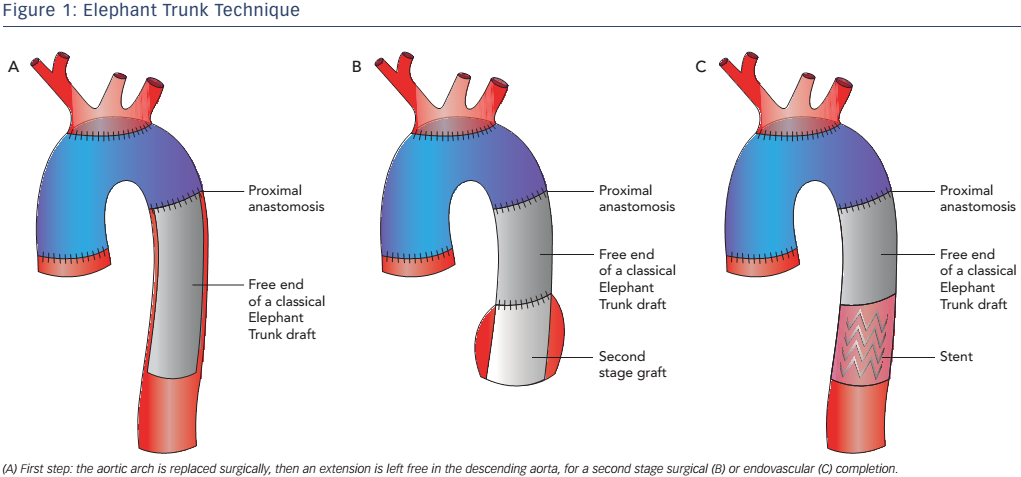
The original ED technique evolved into the frozen elephant trunk (FET), which consists of a hybrid stent-graft prosthesis encompassing a covered stent sutured to the distal end of a conventional tube graft to provide expansive radial force on the distal portion of the ET, allowing a virtual anastomotic seal at the descending aorta4 (see Figure 2). This technique enables the treatment of patients with challenging anatomy and it also allows treatment of disease in the descending aorta at one sitting without the need for subsequent TEVAR. This technique has been used mainly for acute AD (69.8 %) but is also used for aneurysms (30.2 %).10 FET has also been used in chronic AD,12,13 but is only appropriate in selected cases, given the low thrombosis rate of the false lumen in chronic AD, where conventional ET associated with a second intervention on the descending thoracic aorta may be more appropriate.14,15 Despite the benefits offered by FET, it is still associated with considerable mortality and morbidity: reported aggregate 30-day in-hospital mortality is 1.8–17.2 %, while stroke and spinal cord injuries are in the 2.5–20.0 % and 0–21 % ranges, respectively.16-18
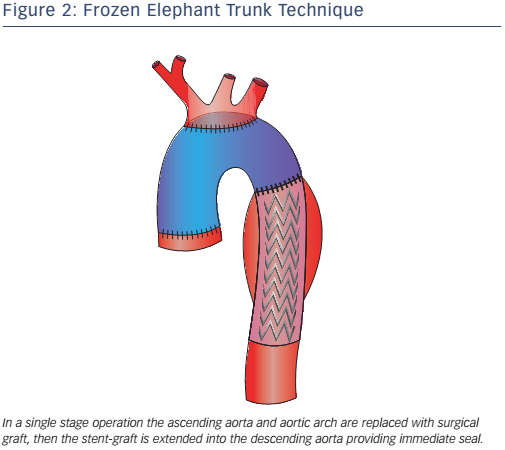
Pochettino et al. further developed FET with the antegrade insertion of a stent-graft into the proximal descending aorta during open type-A dissection repair.15 This hybrid approach, of classic arch replacement with downward placement of a stent-graft through an open arch, has stimulated the development of novel grafts such as the E-vita. The E-vita has a Dacron segment that replaces the ascending aorta and arch, together with a stent graft segment, which is positioned in the proximal descending thoracic aorta. Diagrams of E-vita structure and deployment are illustrated in Figure 3. Initial results from the international E-vita open registry are very promising, with in-hospital mortality of 15 % (40/274), 18 % for acute AD, 13 % for chronic AD and 14 % for thoracic aortic aneurysms.17 New strokes have been observed in 6 % (16/274) and spinal cord injury in 8 % (22/274) of patients.17
Hemi-arch replacement associated with antegrade TEVAR is a surgical technique between conventional ET and FET. It has been used in patients with DeBakey type I AD without an intimal tear in the arch.16 This technique showed no difference in false lumen thrombosis and clinical outcomes with total arch replacement. Nevertheless, compared with total arch replacement, the hemi-arch replacement had a lower incidence of post-operative transient neurological dysfunction (16.9 % versus 30.9 %; p=0.04), low cardiac output syndrome (5.6 % versus 16.7 %; p=0.03) and prolonged ventilation, i.e. >72 hours (7 % versus 19.0 %; p=0.03).19
Revascularisation of the Supra-aortic Branches and TEVAR
Novel hybrid techniques aim to reduce the mortality and morbidity following open total arch replacement requiring deep hypothermia and circulatory arrest, and improve distal organ ischaemia because of the contemporaneous treatment of the descending aorta. Revascularisation techniques essentially bypass graft the supraaortic branches and ligate the native branches (‘debranching’). This is followed by endovascular stent-grafting of the aortic arch with or without subsequent TEVAR of the descending aorta. In ‘partial debranching’ the left carotid artery and subclavian artery are revascularised by carotid–carotid or carotid–subclavian bypass; ‘total debranching’ includes revascularisation of the brachiocephalic trunk. These techniques aim to move the origin of the supra-aortic vessel to zone 0, 1 or 2, according to the type of AD, and allow the TEVAR stent-graft to land whether inserted antegradely or retrogradely. Revascularisation differs with LZ: LZ0 requires all three aortic vessels; LZ1 the LSA and left carotid artery and LZ2 the LSA (some studies advocate the safety of LSA coverage without recanalisation).14,20
Kent et al.21 (one patient) and Chang et al.22 (21 patients) have investigated a new technique based on four-branch grafting for acute type A dissection without the need for circulatory arrest and mild hypothermia. In this technique, the 10-mm side branch is used to deploy an antegrade stent-graft in the descending aorta. Reported in-hospital mortality was 4.8 % and there was no caudal stent-graft migration.21 One type 1 endoleak occurred during the procedure and was repaired with a cuff. Complete false lumen thrombosis was obtained in 85 % of patients during the follow-up period.21
Marullo et al.23 and Esposito et al.24 employed an interesting staged approach whereby the ascending aorta is replaced and the arch debranched with a new multi-branched graft. An endograft is then deployed in the arch and proximal descending thoracic aorta if there is a malperfusion syndrome or early evidence of distal aortic dilation. An endograft was deployed in 62.5 % (15/24)23 and 73 % (65/89)24 of patients in the two studies, respectively, resulting in false lumen thrombosis and no resultant endoleaks. In hospital mortality reported in the two studies was 4.2 % and 8.9 %, respectively.23,24
Total debranching (LZ0) is still associated with higher risk (OR 11.1; 95 % CI [1.86–65.90]; p=0.010) than a minor surgical operation (LZ1 or LZ2),25 although repair is more stable in L0 and LZ1 than LZ2–4.26
Stent-graft Repair without Surgical Supra-aortic Vessel Revascularisation
Originally, treatment of the aortic arch with a stent-graft was difficult because of the lack of a suitable LZ and the need to insert a straight device into a curved structure, leading to a high risk of type I endoleak. To cope with these limitations, branch artery stenting, fenestrated, customised branched stent grafts and multilayer flow modulator stents have been developed.3,20 However, the evolution and application of these stent-grafts has been slower than that of similar devices used in the abdominal aorta, because of the need to avoid occluding the supra-aortic vessels.
Stenting Technique
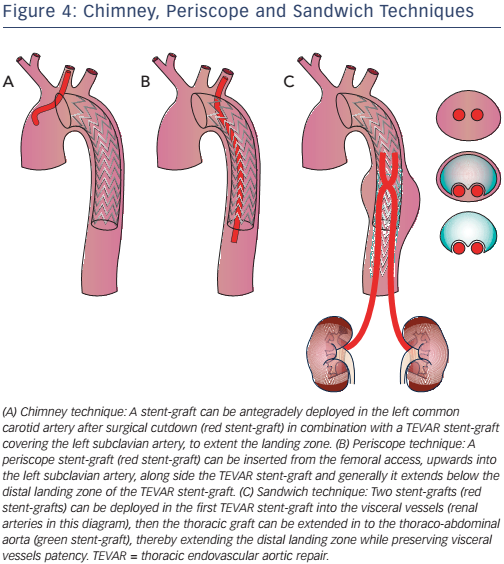
Stenting technique uses parallel stent-grafts as chimneys (that selfexpand when in place), a periscope or sandwiches (see Figure 4) in a variety of aortic pathologies, including acute aortic disease. The use of different types of stents makes this technique easier than fenestrated and branched endografting. High-risk patients presenting in the emergency department with a proximal LZ0 and no other surgical option are suitable candidates for this technique.27,28 Although more than 80 % of cases are successful, drawbacks include chimney stent compression, type I endoleaks and the associated need for re-intervention.27 The classic chimney technique is associated with 10 % mortality and up to 21.6 % occurrence of endoleak (type Ia, 11.8 %), especially in devices positioned in LZ2.29–33
Fenestrated Endografting
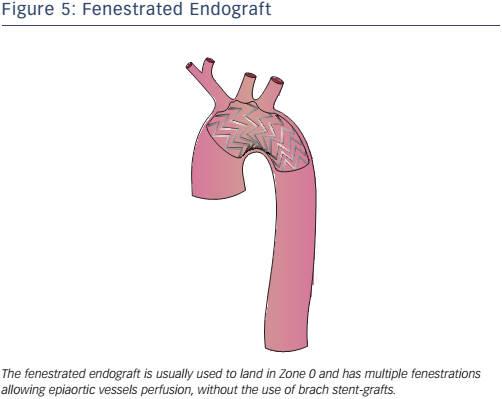
Fenestrated endografting is another recent development. Fenestrated endovascular aortic repair enables the continuation of blood flow to the arteries through fenestrations in the graft (see Figure 5). Deployment of the endograft in the arch is necessary to adjust the fenestrations to correspond to all vessel ostia. Some second-generation devices use new materials, short-length stent elements and an expansion system to adapt to the curvature of the arch. Fenestrated endografts may be pre-curved and have scallops that interrupt the graft, allowing perfusion of the side vessels. The use of pre-curved fenestrated grafts positioned in LZ0–2 is safe and reliable in the treatment of AD and aneurysms involving the aortic arch34 with 95.8 % operative success (defined as the absence of type I or III endoleaks on postoperative computed tomography scan), 1.6 % 30-day mortality and neurological adverse events in 1.8 % of cases.34 Some authors35 have suggested in-situ laser fenestration of the stent-graft followed by a balloonexpandable covered stent to re-perfuse the LSA.
Branched Endografts
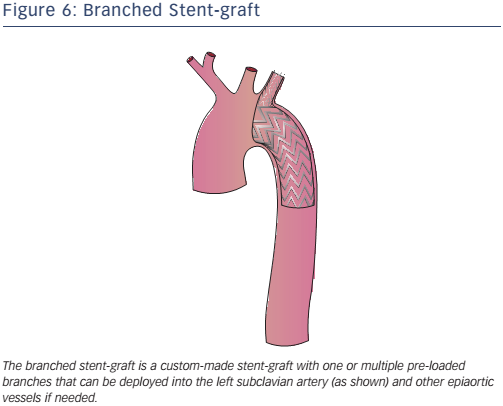
Branched endografts are custom-built, with a lead-time of a few weeks (a delay that may be reduced with the use of 3D printing) and are therefore not suitable for acute aortic pathology (see Figure 6). The presence of built-in branches eliminates the need for adjunctive procedures (hybrid repair, extra-anatomic bypasses and chimneys).
Recently Lu et al. reported excellent mid-term results in patients with type A and B chronic AD: only one patient died from retrograde type A AD and no other complications were reported over the 14–66-month follow-up period.36 There was complete thrombosis of the false lumen in all cases. A trial of Medtronic’s off-the-shelf stent-graft Valiant Mona LSA is recruiting participants and the results are expected in 2021.37 Despite promising initial results, the long-term durability of off-theshelf branched stent grafts is yet to be supported by robust evidence.
Multilayer Flow Modulator Stents
Multilayer stents are a recent solution for the treatment of complex thoracic aortic aneurysm and dissection. They maintain patency of the visceral and spinal vessels (linear flow) while triggering thrombosis of the false lumen/aneurysm sac via turbulent blood flow. Recent studies38,39 in complex thoracoabdominal aneurysms and type B dissection have had high technical success (98.2–100.0 %) and visceral vessels were patent in almost all cases at 1-year (patency >96 %); no cases of spinal cord ischaemia were reported. Although Vaislic et al.39 reported that the aneurysm sac was stable in 18/20 patients at 12 months, in type B AD Sultan et al.38 showed initial remodelling with thrombus formation in the sac/false lumen and an average increase in sac volume of 3.3 % at 12 months. This result was confirmed by the global multilayer modulator registry, which reported a total average increase in sac volume of 5.1 %.40
Evidence of the efficacy of multilayer stents in the AD is still quite weak. Much larger and long-term studies, and preferably a randomised trial against best medical therapy/observation, are required.
Conclusion
Hybrid surgical and endovascular techniques are increasingly being used and developed, improving the morbidity and mortality outcomes for complex patients. The combination of extra-thoracic surgery and TEVAR is currently the most reliable option as it has limited complications and mortality. The endovascular-only approach has shown promising results, but additional long-term studies are warranted.