Patients with severe aortic stenosis who require non-cardiac surgery (NCS) present a difficult clinical problem. It is well established that their rate of postoperative cardiovascular complications is increased in comparison with patients without aortic stenosis,1–3 yet their optimal management remains uncertain. Reported prevalence rates for severe aortic stenosis range from 3.4 % in subjects more than 75 years of age to 18 % in those older than 90 years.4,5 The ageing population together with a greater willingness in recent years to undertake surgical interventions in the elderly means that such patients are faced with increasing frequency. These patients require careful preoperative assessment in order to determine the severity of aortic stenosis, the presence or absence of symptoms and the risks and benefits from NCS. The main challenge, however, is in deciding which patients should undergo aortic valve intervention prior to NCS.
The most recent clinical practice guidelines from the American College of Cardiology/American Heart Association (ACC/AHA)6 and the European Society of Cardiology (ESC)7 for the perioperative cardiovascular assessment and management of patients undergoing NCS were both published in 2014. One of their key aims was to guide decision making regarding aortic valve intervention prior to NCS. This has proven challenging because no trials have investigated whether or not the cumulative risk of aortic valve intervention followed by NCS is lower than the risk of undertaking NCS alone without prior valve intervention. For this reason, both guidelines rely heavily on consensus of opinion (level C evidence), and cite their own society’s valve disease clinical practice guidelines in support of their recommendations. These recommendations deserve closer scrutiny in light of recently published randomised controlled trial data regarding the efficacy of transcatheter aortic valve implantation (TAVI) to treat aortic stenosis.
Clinical Outcomes After NCS in Patients with Aortic Stenosis
Historical series of patients with aortic stenosis who underwent NCS without prior aortic valve intervention reported high rates of postoperative cardiovascular complications. Adverse outcomes were more common with increasing severity of aortic stenosis, with symptomatic rather than asymptomatic aortic stenosis and with increasing complexity of NCS. Among 108 patients with aortic stenosis who underwent NCS at one centre between 1991–2000, for example,3 the rate of perioperative MI or death was 11 % in patients with moderate aortic stenosis (mean gradient 25–49 mmHg) and 31 % in patients with severe aortic stenosis (mean gradient ≥50 mmHg). Aortic stenosis conferred an adjusted OR for perioperative MI or death of 5.2 (95 % CI [1.6–17.0]) in comparison with 216 matched controls. In a more contemporary series of 634 patients who were matched to 2,536 controls,1 death or MI within 30 days after NCS was significantly more frequent in patients with moderate aortic stenosis (4.4 % versus 1.7 %; p=0.002) and severe aortic stenosis (5.7 % versus 2.7 %; p=0.02) compared to controls. Rates of death or MI in patients with symptomatic severe aortic stenosis, asymptomatic severe aortic stenosis and controls were 8.3 %, 4.7 % and 2.7 %, respectively. The difference between controls and study groups was significant in symptomatic patients (p=0.007) but not in asymptomatic patients (p=0.2).
Clinical Practice Guidelines for the Management of Patients Undergoing NCS
Both the ACC/AHA and the ESC guidelines emphasise the importance of general measures in the management of patients with untreated valvular heart disease who are undergoing NCS. In particular, they recommend careful selection of the mode of anaesthesia, use of invasive haemodynamic monitoring (arterial line, transoesophageal echocardiography, etc.), avoidance of rapid changes in volume status, aggressive treatment of arrhythmia and high-intensity postoperative ward care.
ESC Guidelines
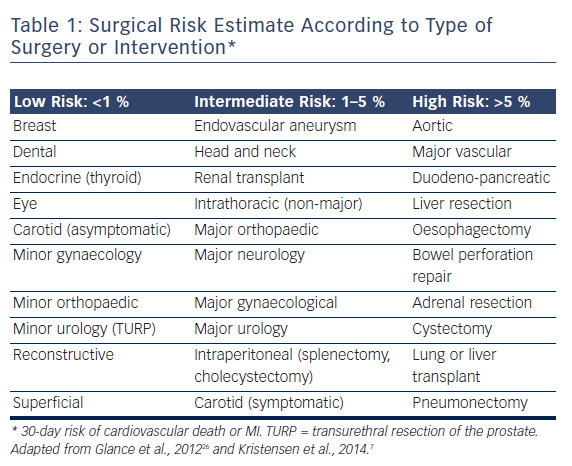
The European guidelines7 are prescriptive with their recommendations for preoperative aortic valve intervention. For patients with symptomatic severe aortic stenosis, aortic valve replacement (AVR) is recommended prior to elective NCS, provided valve surgery would not involve a high risk. In patients with symptomatic severe aortic stenosis for whom AVR would involve a high risk, balloon aortic valvuloplasty (BAV) or, preferably, TAVI “may be a reasonable therapeutic option”. It is recommended that the choice between TAVI and BAV take into account life expectancy and the urgency of NCS. For patients with severe aortic stenosis who are asymptomatic, the recommendations vary according to the NCS risk category (see Table 1). It is recommended AVR be undertaken prior to high-risk NCS, whereas no aortic valve intervention is recommended for patients undergoing low- or intermediate-risk surgery, in whom the risks of NCS are felt to be acceptable.8 If high-risk NCS is planned in patients with asymptomatic severe aortic stenosis who are at high risk for surgical AVR, it is recommended that elective surgery should be performed only if strictly necessary and using invasive haemodynamic monitoring.
ACC/AHA Guidelines
These guidelines6 make the general recommendation that valvular intervention is effective in reducing perioperative risk in adults who meet standard indications for valvular intervention (replacement or repair) on the basis of symptoms and severity (Class I recommendation, level of evidence C). Specifically, they recommend that patients who have symptomatic severe aortic stenosis should undergo AVR before elective NCS. For patients with aortic stenosis who meet indications for AVR but are considered high risk or ineligible for AVR the guidelines note there are three options: undertaking NCS with untreated aortic stenosis, BAV and TAVI. The ACC/AHA guidelines state it is reasonable to perform elevated (moderate) risk elective NCS in patients with asymptomatic severe aortic stenosis with appropriate intraoperative and postoperative haemodynamic monitoring.
Procedural mortality rates of 2–3 % and stroke rates of 1–2 % are reported in the literature relating to BAV,9,10 and small case series of patients with aortic stenosis who underwent NCS after BAV report low rates of postsurgical complications.11,12 The unpredictable effect of BAV on the severity of aortic stenosis and a restenosis rate of 50 % at 6 months, in combination with reducing vascular access sheath sizes for TAVI, however, make BAV an unattractive treatment option for most patients with aortic stenosis. Nonetheless, procedures are short, they require little technical expertise or expensive equipment, they rarely cause conduction disorders and they can be organised quickly. As a result, BAV remains a useful intervention prior to NCS in patients with severe aortic stenosis who require urgent surgery or who have an uncertain long-term prognosis.
In discussing the potential use of TAVI, the ACC/AHA guidelines cite the 1-year mortality rates from the first Placement of Aortic Transcatheter Valves (PARTNER) trial: 24.2 % and 26.8 % for TAVI and AVR in cohort A, 30.7 % and 50.7 % for TAVI and medical treatment in cohort B.13,14 Perhaps more pertinent would have been the 30-day outcomes. In PARTNER A the 30-day mortality was 3.4 % following TAVI and 6.5 % following AVR (p=0.07). For the transfemoral cohort the “as treated” figures were 3.7 % and 8.2 % (p=0.046). Mean length of stay was 8 and 12 days (p<0.001) following TAVI and AVR, respectively. The guidelines go on to note there are no data that demonstrate the efficacy or safety of TAVI for patients with aortic stenosis who are undergoing NCS. Equally, however, there are no such data for surgical AVR.
Contemporary TAVI Data
Three important randomised controlled trials and new observational data regarding the use of TAVI to treat severe aortic stenosis have been published since the clinical practice guidelines on preoperative management were developed.
In a randomised controlled trial of 795 high-risk patients with an average Society of Thoracic Surgeons (STS) score of 7.4 %, TAVI using the Medtronic CoreValve® (Medtronic) self-expanding prosthesis achieved superior clinical outcomes to AVR.15,16 All-cause mortality rates for TAVI compared with AVR at 1- and 2-year follow up were 14.2 % versus 19.1 % (p=0.04) and 22.2 % versus 28.6 % (p<0.05), respectively, while 1-year stroke rates were 8.8 % in TAVI patients compared with 12.6 % in the surgical group (p=0.1).
In the Nordic Aortic Valve Intervention (NOTION) trial, equivalent 1-year clinical outcomes following TAVI and AVR were demonstrated in 280 low-risk patients (mean STS score 3 %).17 Rates of all-cause mortality were 4.9 % and 7.5 % (p=0.38), while stroke rates were 2.9 % and 4.6 % (p=0.44) following TAVI and surgical AVR, respectively.
The PARTNER 2a trial, which studied an intermediate-risk population for AVR (mean STS score 5.8 %), was published in April 2016.18 The combined incidence of death or stroke was 6.1 % versus 8.0 % (p=0.11) at 30 days and 14.5 % versus 16.4 % (p=0.24) 1 year after TAVI and AVR, respectively. Event rates were lowest in patients who were randomly allocated to undergo TAVI via the transfemoral route (12.3 % at 1 year) and were significantly lower in these patients than in patients who were allocated to undergo surgical AVR (15.9 %; p=0.05). The median length of postprocedure hospital stay was significantly shorter following TAVI than it was following AVR (6 days versus 9 days; p<0.001). Aortic regurgitation of moderate or greater severity was significantly (p<0.001) less common after AVR than after TAVI at all time points. The PARTNER 2a trial, however, was undertaken using the now semi-obsolete second-generation SAPIEN XT prosthesis (Edwards Lifesciences). Nonrandomised data have consistently demonstrated better results in cases utilising the third-generation SAPIEN 3 (Edwards Lifesciences) prosthesis, with 1-year rates of paravalvular leak (PVL) and mortality of 2.0 % and 6.5 %, respectively, in intermediate-risk patients.19
Finally, registry data relating to the Lotus valve (Boston Scientific) from a small cohort of 112 patients at high risk for AVR (average STS score 7.1 %) demonstrated moderate or more severe PVL in only 1 % of cases at 30 days with death or disabling stroke occurring in 5.9 %.20
Coronary Artery Disease and DAPT
The clinical practice guidelines from the ACC/AHA21 and the ESC22 for the management of patients with valvular heart disease recommend that significant (>70 % reduction in luminal diameter) coronary artery disease be treated with coronary artery bypass grafting (CABG) undertaken at the time of AVR (level of evidence C). The guidelines for the management of patients undergoing NCS defer to the valvular heart disease guidelines in respect to this issue. These guidelines make no recommendations for the management of coronary artery disease in patients undergoing TAVI, other than to state untreated coronary artery disease is a relative contraindication to TAVI, particularly in those with severe multivessel coronary artery disease. What is clear, however, is that dual antiplatelet therapy is required after percutaneous coronary artery intervention (PCI), but not necessarily required after combined CABG and AVR operations or after TAVI undertaken without prior coronary intervention. Dual antiplatelet therapy is commonly prescribed after TAVI,13,14,18 but there are no evidence-based guidelines to support their use and in general operators do not have the concerns about cessation of antiplatelet therapy after TAVI that they do after PCI. Thus, the presence of significant coronary artery disease is an important factor when deciding which form of aortic valve intervention should be undertaken prior to any NCS that cannot safely be performed in patients on antiplatelet therapy.
Translating Evidence into Practice
The main evidence for TAVI prior to the publication of the current clinical practice guidelines for the perioperative management of patients undergoing NCS came from the first PARTNER trial. This showed that TAVI is at least as effective as AVR in patients with severe aortic stenosis who are at high risk of complications from AVR. Since then, further data have been published that have consolidated the role of TAVI in high-risk patients and show that TAVI is also safe and effective in intermediate-risk patients. Current guidelines recommend that severe aortic stenosis is treated prior to all forms of NCS in symptomatic patients. European guidelines recommend that severe aortic stenosis is also treated in asymptomatic patients who are scheduled to undergo high-risk NCS. TAVI is recommended as an option prior to high-risk NCS only in symptomatic patients who are at high risk from AVR. TAVI is not mentioned as a treatment option for asymptomatic patients. In the context of patients who require treatment for aortic stenosis prior to NCS, however, TAVI has several advantages over AVR: the procedural mortality rate is lower in highand intermediate-risk patients, it is less invasive and the recovery time is quicker, which facilitates early NCS after the procedure. In addition, the latest iterations of TAVI prostheses require smaller sheath sizes promising further reductions in length of stay, with the potential for next-day discharge in some cases, and lower rates of PVL allay some of the concerns relating to long-term outcomes following TAVI.
In recent years, the “Heart Team” has emerged as the key to clinical decision making for complex cardiac patients.23 Interventional cardiologists and cardiac surgeons who are directly acquainted with the relative merits of the different forms of intervention have become expert in assessing and managing patients with aortic stenosis.24,25 The ACC/ AHA guidelines for the management of valve disease21 give considerable focus to the Heart Valve Team for determining which patients with aortic stenosis should be treated by conventional AVR and which by TAVR. It is, therefore, curious that Heart Team-directed management is not recommended for the management of patients with aortic stenosis in the perioperative setting. Guidelines can provide useful recommendations for the management of single pathologies but evidence relating to the management of dual pathologies is necessarily sparse and it is in this context that the Heart Team is particularly valuable, more so in patients with a third pathology in the form of concomitant coronary artery disease.
Conclusion
The next iterations of clinical practice guidelines for perioperative management of patients undergoing NCS should recognise the benefits of TAVI compared with conventional AVR in treating patients with severe aortic stenosis. When aortic valve intervention is indicated, the Heart Valve Team is best placed to decide which intervention is most appropriate.