In the 18th century, Edward Jenner and Caleb Hillier Parry, two British physicians, independently noticed that coronary ossification was often present in patients dying of ‘syncope anginosa’.1 Coronary angiography opened the possibility of expanding these observations to living patients and offered revascularisation options. During surgery, it was often possible to modify the position of the distal coronary anastomoses to avoid calcified segments. In terms of percutaneous treatment, heavily calcified coronary lesions still represent a challenge for interventional cardiology, with a greater risk of immediate complications and late failure due to stent underexpansion and malapposition.2 Good characterisation of calcium distribution with multimodal imaging and optimal lesion preparation, facilitated by the introduction of new dedicated devices, are essential for successful treatment of calcified coronary stenoses.3,4
Pathophysiology of the Calcified Coronary Plaque
When discussing artery calcification, it is appropriate to distinguish between peripheral and coronary calcifications. In the peripheral arteries of the lower extremities, medial calcification is often found, driven by the action of osteoblast-like cells and due to various factors, such as hypercalcaemia, high phosphate blood concentrations and increased parathyroid hormone concentrations.
Conversely, the mechanism underlying the development of atherosclerotic coronary calcification is different, with dysmorphic calcium precipitation driven by chondrocyte-like cells and linked to expression of inflammatory factors, such as cytokines released by tissue macrophages and foam cells.5 It is likely that inflammation precedes calcification and plays an important role in its progression, with the two processes coexisting and promoting each other.
The primary cause of the calcification process in atheroma is the death of inflammatory cells and smooth muscle cells (SMCs), with macrophage-derived matrix vesicles also playing a role in this event. An intense inflammatory response due to cholesterol deposits caught under the endothelium leads to the development of microcalcification over areas ranging in size between 0.5 and 15.0 mm.6 The differentiation of several cell types (e.g. pericytes and vascular SMCs) promotes bone deposition and contributes to necrotic core formation because of the local degradation of collagen fibres.7
The entire process is driven by the fusion of cell debris originating from SMC apoptosis, which then serves as a focus for calcium phosphate crystal formation. A lack of calcification inhibitory factors (e.g. matrix gamma-carboxyglutamic acid protein, pyrophosphate, fetuin-A, osteopontin and osteoprotegerin) also plays a role in the imbalance between osteogenic and osteoclastic mechanisms.8 Some drugs, such as phosphate binders or receptor activator of nuclear factor-kappaB ligand blockers, are under investigation to reverse or prevent vascular calcification.7
Microcalcifications can only be detected histopathologically using special stains, such as von Kossa stain and Alizarin red. Microcalcifications can coalesce into larger masses over time to form speckles and calcified sheets, detectable in vivo using CT or intravascular imaging. In addition to plaque rupture and erosion, the development of subintimal large protruding masses may be a possible cause of plaque destabilisation and thrombus formation, and has been detected in 5% of cases of unstable syndrome and ST-elevation MI. This role of the protruding masses in plaque destabilisation is at odds with the common belief that the substitution of large necrotic cores in positively remodelled arteries with fibrocalcific tissue, also promoted by statin therapy, is a marker of plaque stabilisation.6,9
Coronary Calcification as a Predictor of Events
CT allows calculation of the coronary artery calcium score, an independent predictor of coronary events in both asymptomatic and symptomatic individuals, as confirmed by Budoff et al. in the 10-year results of the Multi-Ethnic Study of Atherosclerosis (MESA) study.10–14 Spotty calcifications are associated with unstable plaques and acute coronary syndrome.15
Coronary calcification is also a marker of advanced atherosclerosis and is correlated with multivessel coronary disease and the presence of complex lesions, including long lesions, chronic total occlusions and bifurcations. The SYNTAX score is a method of estimating the complexity of coronary disease. The location and severity of lesions are the main drivers of high scores. Heavy calcification, visible with fluoroscopy, is also considered, adding 2 points per lesion.16–19
In recent registries and meta-analyses, the prevalence of moderate and severe calcific coronary stenoses has been reported to range between 18% and 26%.16–18 In these studies, severe calcific lesions were found to be associated with advanced age, systemic hypertension, dyslipidaemia and diabetes. Chronic kidney disease can also be associated with severe coronary calcification. As a consequence of a progressively aging population and an increase in these comorbidities, we expect a higher frequency of severe coronary calcification in patients undergoing percutaneous coronary intervention (PCI) in coming years.
PCI of coronary calcified lesions is associated with major stent underexpansion and malapposition, with major periprocedural complications and, subsequently, a high rate of target lesion failure, restenosis or thrombosis. Consequently, calcified coronary lesions are a predictor of a worse clinical outcome, associated with higher mortality, major adverse cardiovascular events (MACE) and target vessel failure on multivariate analysis after correction for confounders.19–21 These results were confirmed in a recent registry,17 as well as in a pooled analysis in patients treated exclusively with a second-generation drug-eluting stent (DES).18 Late and very late stent thrombosis are also more frequent in patients with calcific stenosis.18–20
Calcium and Imaging Techniques
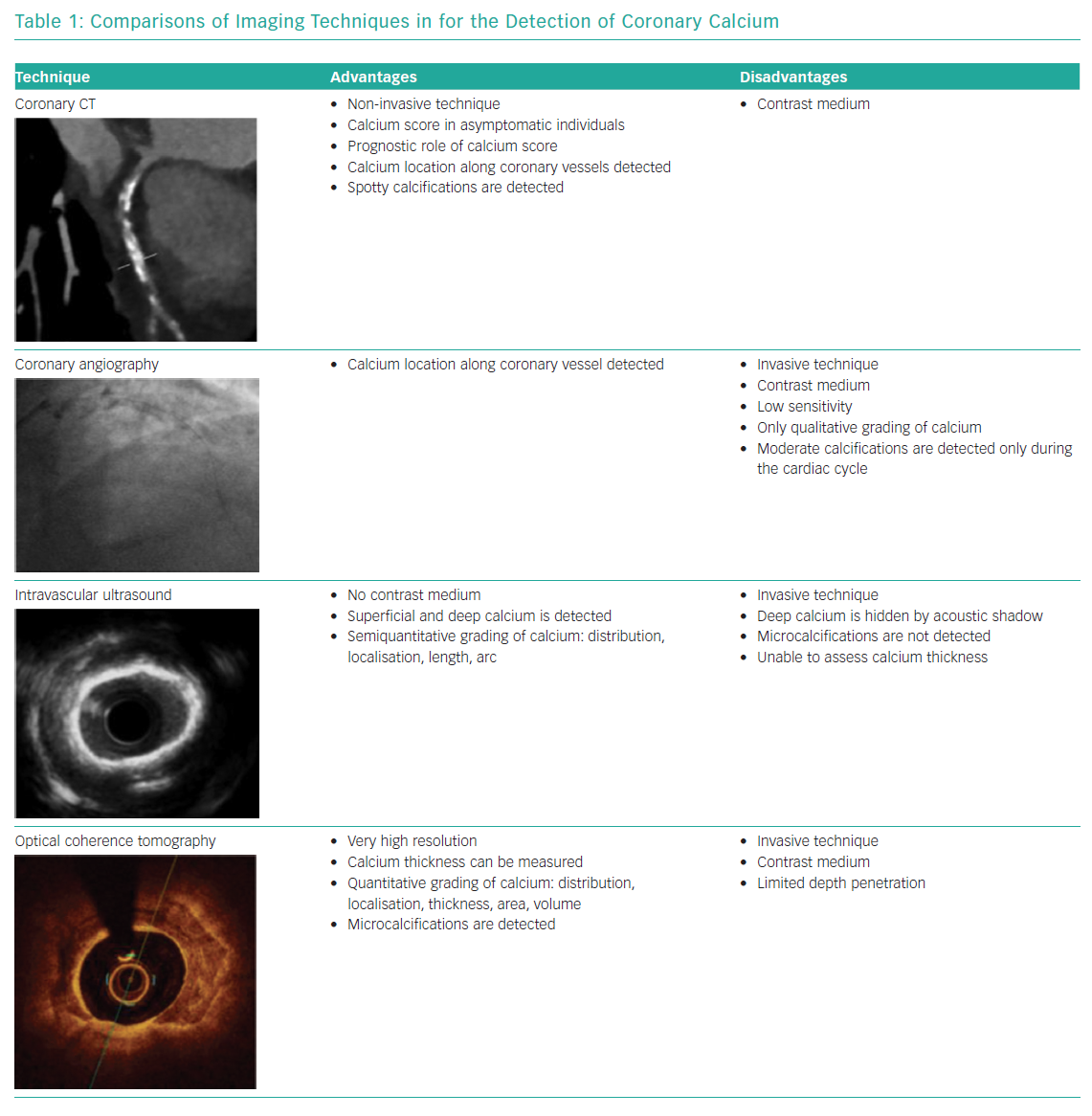
Coronary CT angiography (CCTA) is the most important non-invasive imaging technique used to detect calcium. Calcium is detected as an area of hyperattenuation, defined as an area of at least 1 mm2 with >130 Hounsfield units or ≥3 adjacent pixels using the Agatston method.22 The calcium score is the sum of all coronary calcified lesions (excluding calcium in the valve or aorta) and is a strong prognostic factor for clinical events in the mid to long term in asymptomatic individuals.11,14,15 CCTA is able to characterise coronary plaques; in particular, CCTA can detect spotty calcification, which is one of the four signs of vulnerable plaques (i.e. low CT attenuation, remarkable positive remodelling, spotty calcification and the napkin-ring sign).23 CCTA is also a valuable tool in planning PCI because it allows accurate identification of calcium in coronary lesions and localisation of calcium along coronary vessels, improving procedural success.
Coronary angiography often underestimates calcium, its grading is not accurate and calcium depth within the plaque is not assessed.4 Moderate calcification is defined as radiopacity observed only during the cardiac cycle before injection of contrast medium. Severe calcification is defined as radiopacity observed without cardiac motion, visible on both sides of the arterial lumen, as a double track. Wang et al. assessed 440 lesions with fluoroscopy, intravascular ultrasound (IVUS) and optical coherence tomography (OCT) and showed that IVUS was a particularly sensitive method of detection; in fact, calcium was detected by angiography in 40.2% of lesions, by IVUS in 82.7% of lesions and by OCT in 76.8% of lesions.4
On IVUS, calcium is immediately apparent as bright, hyperechoic lines with acoustic shadowing; deep calcium can also be detected using IVUS because of the high penetration power of ultrasound.3 Calcified plaques can be assessed semiquantitatively, with calcium arc and calcium length, using this technique; but the thickness of calcium and microcalcifications cannot be detected. Semiquantitative assessment of calcified plaques with IVUS allows the estimation of calcific burden; in particular, maximum circumferential extension of calcium >180° is associated with a smaller stent area and greater stent eccentricity.24
OCT features of calcium are subtler, with a low-intensity signal area and well-delineated external contours. OCT can measure calcium thickness and shows calcium disruption better because of its higher resolution, but may miss deep calcifications due to insufficient penetration. With OCT one can perform quantitative assessments of calcified plaques (i.e. calcium arc, length, thickness, area and volume) and detect microcalficiations.25 Fujino et al. recently proposed an OCT score to grade calcium plaques with a numerical cut-off; calcium arc >180° (2 points), calcium length >5 mm (1 point) and calcium thickness >0.5 mm (1 point) were associated with poor stent expansion (stent expansion <70%).26
IVUS and OCT are two invasive techniques that allow us to better characterise the depth and distribution of calcium in coronary plaques (Table 1).
Percutaneous Coronary Intervention of Complex Calcified Coronary Lesions
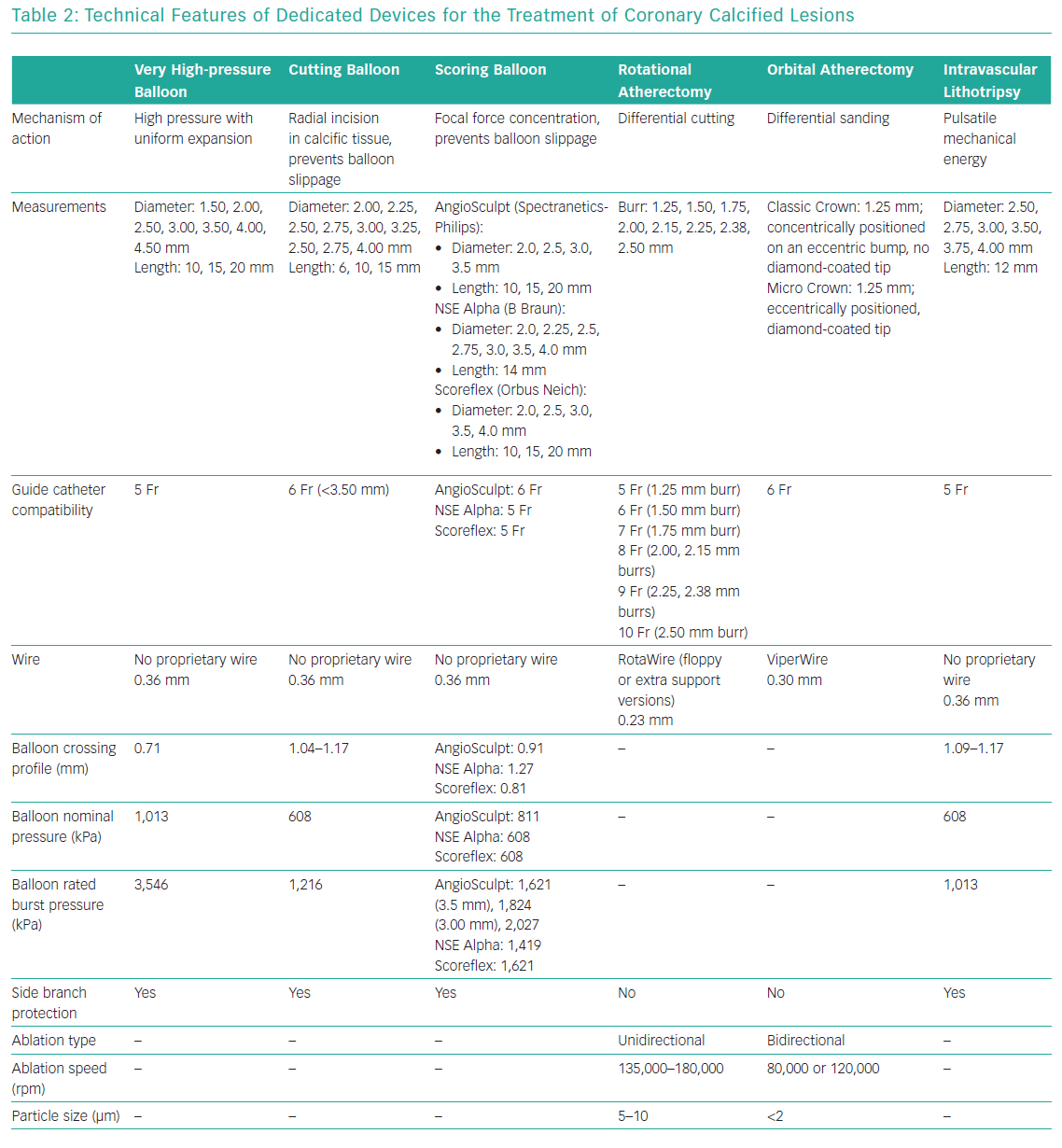
Either a transfemoral or transradial approach can be used in PCI for heavily calcified lesions, with the choice depending primarily on operator experience. Support wires, buddy wires, guide extensions, lesion predilatation and anchoring of the guide catheter with inflation of a second balloon in a side branch or distal vessel are possible options for the treatment of calcified coronary stenoses.27,28 When successful treatment cannot be obtained using these options, a dedicated device should be used for adequate lesion preparation (Table 2).29
High- and Very High-pressure Non-compliant Balloons
Non-compliant (NC) balloons, unlike semicompliant balloons, tolerate high inflation pressures, exhibiting a small increase in diameter. NC balloons allow more uniform balloon expansion and the application of higher forces in a focal segment of a coronary vessel, avoiding dog-bone deformation exerting excessive pressure at the edges and potentially causing coronary dissections or perforations.29,30 Repeated and prolonged inflations with NC balloons should be encouraged as the first choice in mild to moderate calcified stenoses, where the calcium arc is restricted (<90°).
The OPN NC balloon (SIS Medical) uses twin-layer technology to allow super high pressures within the balloon with minimal increases in diameter. This balloon can be inflated with a nominal pressure of 1,010 kPa and a rated burst pressure of 3,546 kPa, but the balloon was tested up to a pressure of 4,560 kPa and many operators report very rare balloon ruptures at pressures as high as 5,573 kPa, the maximal level allowed by the special indeflator provided with the balloon. The OPN NC balloon is available in diameters ranging from 1.5 to 4.5 mm (in 0.5 mm increments) and lengths of 10, 15 and 20 mm. The OPN NC balloon is a dedicated device for the treatment of in-stent restenosis, heavily calcified lesions or other lesions that cannot be dilated. In the past, it was often used for lesion preparation before bioresorbable vascular scaffold (BVS) implantation or to expand the BVS fully, given the small increases in diameter (<0.5 mm) stated by the manufacturer.31,32
In restricted clinical experience, the OPN NC balloon successfully treated >90% of undilatable lesions compared with a conventional NC balloon with a 0.9% rate of coronary rupture.33 The OPN balloon is compatible with 0.014 inch wires and 5 Fr access, but it has a high profile (0.028 inches), although this is better than scoring and cutting balloons, and so it is difficult to recross or reuse after inflation.
Cutting and Scoring Balloons
The Flextome Cutting Balloon (Boston Scientific), introduced in 1991, is available in monorail or over-the-wire catheter, with the most recent iteration (Wolverine) improved in terms of profile and flexibility. The Flextome Cutting Balloon is available in diameter sizes ranging from 2.0 to 4.0 mm (in 0.25 mm increments) and lengths of 6, 10 or 15 mm.34 On a cutting balloon, three or four metal microblades are placed longitudinally on the surface of the balloon and the balloon works by cutting the media with radial incisions, thus reducing elastic recoil and minimising neointima proliferation. The microblades also prevent balloon slippage, which is particularly helpful for in-stent restenosis due to intimal hyperplasia.35 Initial clinical experience with cutting balloon angioplasty appeared favourable because it achieved more lumen enlargement compared with traditional balloon angioplasty.35 However, a later large randomised trial showed similar acute procedural success in patients with de novo undilatable lesions treated with cutting balloon or conventional balloon angioplasty, a similar binary restenosis rate at 6 months between the two groups (31.4% versus 30.4%, respectively; p=0.75) and a significantly higher perforation rate in the cutting angioplasty group (0.8% versus 0%; p=0.03).36
These unfavourable clinical results, the large use of coronary stents and the very high crossing profile of cutting balloons (0.041–0.046 inches) have discouraged their use in current clinical practice, with a restricted application to resistant lesions in the European Society of Cardiology and American College of Cardiology guidelines.37,38
Scoring balloons are semicompliant balloons encircled by scoring elements. These scoring elements allow focal concentration of the force during inflation and decrease balloon slippage. Scoring balloons have similar indications to cutting balloons, but scoring balloons are more flexible, have a better profile and can achieve a full expansion with a low inflation pressure, with consequently less trauma to vessel walls and a minor risk of coronary dissections.39,40
Several types of scoring balloons are now available for treatment of mild to moderate calcified lesions. The AngioSculpt (Spectranetics-Philips) is a semicompliant balloon with three spiral rectangular Nitinol scoring elements, also available in a drug-coated version (AngioSculpt X, Spectranetics-Philips).41,42 In a feasibility trial, the AngioSculpt balloon was used for the treatment of de novo lesions prior to BMS implantation and showed very high procedural success and a target lesion revascularisation rate of 10% at 6 months.43
These results were confirmed in an observational study, in which 37 patients treated with AngioSculpt before stent implantation were compared to 145 patients treated with direct stenting and 117 patients with traditional plain old balloon angioplasty before stent implantation. IVUS assessment showed greater stent expansion in the AngioSculpt group than in the other two groups (89% versus 74% of vessels with an area >5.0 mm2, respectively).44
The NSE Alpha scoring balloon (B Braun) has three triangular flexible nylon elements on the balloon surface attached only at the proximal and distal edges of the balloon. Promising results for predilatation of severe calcified lesions were shown with the leopard-crawl technique.45 The Scoreflex (Orbus Neich) is a semicompliant balloon with two fixed Nitinol wires on opposite sides of the balloon surface.46 Otsuka et al. reported a case series where prolonged inflation of the Scoreflex balloon allowed adequate dilation of severe calcified plaques as shown by the ‘creep phenomenon’, whereby prolonged inflation of the balloon produces a distortion force capable of expanding a resistant calcified lesion.47 Scoring balloons have been considered by cardiologists as an alternative to cutting balloons and, in recent years, have been preferred because of major flexibility and deliverability, although no specific randomised control trials are reported in the literature.
In the PREPARE Severely CALCified Coronary Lesions Trial (PREPARE-CALC), 200 patients were randomised 1:1 to treatment with either rotational atherectomy (RA) or modified balloons (both cutting and scoring balloons). Lesions treated with the modified balloons had similar late lumen loss at 9 months compared with RA, but high crossover was reported in the study (16% of lesions were untreatable with modified balloons).48
Rotational Atherectomy
RA was introduced more than 30 years ago to debulk and modify atherosclerotic plaques as an alternative or adjunctive strategy to percutaneous balloon angioplasty.49 Although initial positive experiences with RA showed short-term lumen enlargement, there was also a high rate of target lesion revascularisation due to cell proliferation and restenosis.50 In the DES era, in-stent restenosis decreased markedly; consequently, RA was reserved for lesion preparation before stent implantation in the case of heavily calcified stenosis, as confirmed by Society for Cardiovascular Angiography and Interventions guidelines in 2011 (Level IIa evidence, Class C recommendation).51 The rotating burr can selectively ablate inelastic calcific atheroma, sparing elastic vascular tissue, but it can also generate microparticles (5–10 µm) that propagate distally in the coronary vessel, with consequent vascular plugging, slow flow or no reflow and increases in myocardial-specific enzymes.52 IVUS analysis after RA showed lumen enlargement with ‘bites’ in the calcific plaque.53 OCT analysis showed plaque modification with a new, larger, smooth lumen vessel created from the rotating burr and fissures or craters in the intima or intima–media wall.54,55
In a Cochrane review by Wasiak et al. of 12 trials published between 1996 and 2005 and enrolling 3,474 patients, there were no significant differences in restenosis or major adverse cardiac events (MACE) at 6 months or 1 year after the treatment of complex lesions with RA or percutaneous transluminal coronary angioplasty alone, but RA was associated with a higher periprocedural complication rate.56
More recently in the Rotational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease (ROTAXUS) trial, 240 patients with calcified lesions were randomised to RA before stenting or stenting alone (paclitaxel stent).57 This trial showed higher procedural success in the RA group (92.5% versus 83.3%; p=0.03) and better acute lumen enlargement, but a significantly higher late lumen loss at 9 months, although the trial is limited by an 8% crossover rate and the exclusion of more severe calcified lesions.57
Encouraging results have emerged from PREPARE-CALC, published in 2018, in which 200 patients were randomised to treatment with either RA or cutting or scoring balloons.48 In that study, a greater success rate was achieved with RA than with modified balloons (98% versus 81%, respectively), driven primarily by delivery failure of the bulky cutting or scoring balloons, with no significant difference in late lumen loss at 9 months.48 Periprocedural complications were low and similar in the two groups, probably due to highly experienced operators.48 RA should be used in combination with cutting balloons, as reported by Amemiya et al. in single-centre observation study with good results in terms of the number and thickness of calcium fractures, assessed with OCT and stent expansion.58
The Rotablator System (Boston Scientific) is made up of three components: a nickel-plated elliptic burr coated with diamond microscopic crystals that is available in sizes ranging from 1.25 to 2.50 mm diameter; a single advancer that can transmit rotational speed to the burr and is connected with a gas-driven turbine; and a control console and foot pedal (in most recent models, the latter has been replaced by an activator in the connecting handle).59 In recent years, a reduction in burr size and a more standardised protocol with low rotation speeds have decreased periprocedural complications.27,60 A transradial approach is allowed with 1.25, 1.50 or 1.75 mm burrs. An ultrathin (0.009 inch) steerable dedicated guidewire (RotaWire) of length 330 mm is used to cross the calcified lesion; it is available in a floppy version or an extra support version, useful primarily in the treatment of aorto-ostial lesions. These dedicated wires are less torque responsive and more steerable; furthermore, a regular 0.014 inch guidewire can be used to cross the stenosis and the RotaWire can then be inserted through an over-the-wire balloon or microcatheter. The RotaWire must be placed in the main vessel and other guidewires have to be removed from side branches to avoid wire cutting or perforation.
When the burr is proximal to the lesion, rotablation can be started with short burr runs (<20 seconds) at low rotational speed (135,000–180,000 rpm). Small burrs (1.25 and 1.50 mm; burr-to-artery ratio=0.6) are adequate in most lesions as an initial approach, but occasionally it may be necessary to increase burr size with a step-up approach. Fluoroscopic, acoustic and tactile signals should be monitored to avoid significant deceleration in rotational speed (>5,000 rpm), which is associated with complications. Possible complications of RA are no reflow or slow flow, treatable with a pharmacological strategy based on intracoronary vasodilators (nitroprusside, adenosine, nicorandil), burr entrapment, coronary perforations and transient atrioventricular block.61 Prophylactic insertion of a temporary ventricular pacemaker can be considered for long right coronary artery lesions, but atropine before starting burr activation is sufficient to prevent the problem in most other cases. RA is contraindicated in saphenous vein graft stenosis, dissection and in thrombosis, even if a large registry of 1,308 patients with acute coronary syndrome showed the feasibility of RA in this clinical setting.62
Although RA is considered the gold standard technique to prepare heavily calcified lesions before stent implantation, particularly when lesions are uncrossable with a balloon device, and it appears to have become safer in recent years, it is used only in 1–3% of PCIs in Europe and is limited to expert operators in high-volume centres,60 probably because of potential complications as well as the costs, which are not negligible, or low insurance recruitment.
Orbital Atherectomy
The Diamondback 360 Coronary Orbital Atherectomy (OA) System (Cardiovascular Systems) was approved by the US Food and Drug Administration in 2013 for the treatment of de novo severe calcified coronary stenosis. This device uses the elliptical movement of a single-sized, eccentrically mounted crown to create centrifugal force, differentially sanding the hard component of the plaque and sparing the soft tissue component.63 Comparing OA and RA highlights differences in structural components, mechanisms of action and different anatomical settings in which to use these devices:64–66
- A single-sized crown of 1.25 mm is used in OA to treat vessels with a diameter of 2.5–4.0 mm, regulating rotational speed.
- Two rotational speeds, namely 80,000 rpm (low speed) and 120,000 rpm (high speed), allow different ablation of vessels with different diameters, with the high speed creating greater centrifugal force and a higher orbital diameter.
- In OA, the crown rotation is less concentric; therefore, in aorto-ostial lesions, where there is a large transition from large to small lumen, OA should be used with caution.
- ViperWire Advance (Cardiovascular Systems) is a dedicated wire for the Diamondback 360 Coronary OA System and is available in one version only (no floppy or extra support versions, as available for the RotaWire) and has 0.012 inch profile.
- In OA, a bidirectional atherectomy is performed, not only anterograde as in RA, with a consequent decrease in crown entrapment.
- Continuous flow of blood and saline solution or other lubricant solutions (e.g. soybean oil, egg yolk) during ablation minimises thermal injury and potentially decreases no-reflow and periprocedural complications.
- The smaller microparticles created by OA (2 µm) have a minimal effect on the microcirculation.
- Device activation is simpler and faster for OA than RA (one-touch activation and 2 minutes, respectively).
Some innovative components of the OA system are now available for clinical use in Japan or for research applications. The Coronary OA Micro Crown (Cardiovascular Systems) has a new diamond-coated tip to better reach the target lesion and a 1.25 mm eccentric crown that allows it to rotate at lower speeds and create an orbit similar to the Classic Crown, which, instead, is located concentrically on an eccentric bump. In addition, a new ViperWire and GlideAssist system are available to easily track the OA device through tortuous coronary vessels.
OCT has been used to demonstrate the mechanism of action of OA, revealing deeper and longer cuts in calcific plaques with OA than RA. In addition, OA modified calcified plaques more than RA, with consequently better stent apposition and expansion.54 A mean ablation area of 0.55 ± 0.41 mm2 and an ablation volume of 2.68 ± 2.80 mm3 were measured with OCT in a case series of 18 patients, with 18% of intimal dissections reported.67
The Orbital Atherectomy System in treating de novo calcified coronary lesions (ORBIT I) study was the first-in-human trial to evaluate the safety and performance of OA in 50 patients at two Indian centres, showing a procedural success rate of 94% and a MACE rate of 8% at 30 days.68 The ORBIT II study is a non-randomised trial, enrolling 443 patients with severe calcified lesion in 49 US hospitals.69 ORBIT II showed high procedural success (98.6% of patients with <50% residual stenosis) and good outcomes, namely 4.4% cardiac deaths at 2 years, 8.1% target lesion revascularisation at 2 years and low periprocedural complications (slow flow <1%, coronary dissections 5.9%). Lee et al. reported real-world data for 458 patients treated with OA in three US hospitals, confirming positive results at 1 year.70
The Diamondback 360 Micro Crown has recently been proposed as a technological advancement; it is able to obtain a higher sanding area with lower rotational speed (50,000–70,000 rpm). This new device was used in the Coronary Orbital Atherectomy System Study (COAST), which found that 85% of patients were free of MACE at 30 days.71 The Evaluation of Treatment Strategies for Severe CaLcIfic Coronary Arteries: Orbital Atherectomy vs. Conventional Angioplasty Technique Prior to Implantation of Drug-Eluting StEnts: The ECLIPSE Trial (ECLIPSE; NCT03108456), a large on-going trial in which approximately 2,000 patients with severe calcified lesions will be enrolled to OA or traditional angioplasty groups, will provide scientific evidence regarding OA.72 The Feasibility of Orbital Atherectomy System in Calcified Bifurcation Lesion (ORBID-OA) study is an on-going US observational study in which 30 patients will be assessed with frequency domain OCT.73 While waiting for the results of these trials, new data have emerged from the Manufacturer and User Facility Device Experience (MAUDE) database of a not negligible number of cases of perforations attributed primarily to excessive straightening from the Viper wire and prompting changes in technique.74
Excimer Laser Coronary Atherectomy
Excimer laser coronary atherectomy (ELCA) modifies calcified plaques using photochemical and photothermal ablation. The CVX 300 System (Philips) uses xenon chloride to produce a light emitted in the ultraviolet B spectrum (308 nm) with a penetration depth of 30–50 µm to limit medial and adventitia damage. Microparticles (<10 µm) released have a low impact on the microcirculation.75 ELCA catheters are available in four diameters, which are compatible with 6, 7 and 8 Fr catheters; 6 Fr: 0.9 and 1.4, 7 Fr: 1.7 and 8 Fr: 2.0 mm, based on a catheter:vessel diameter ratio of 0.5:0.6, and are compatible with a 0.014 inch guidewire. Initial experience with ELCA treatment for complex coronary artery lesions reported a procedural success rate of 93% and both minor and major periprocedural complications.76,77
In the context of coronary calcified lesions, the use of ELCA is limited to that of a ‘bail-out’ strategy, when lesions are uncrossable for dedicated balloons or for the RotaWire or ViperWire, and its use is limited to a restricted number of centres. A combination of ELCA and RA was recently described (the RASER technique).78 The main application of this technique remains in the treatment of severe in-stent restenosis, with positive results reported in a recent OCT study.79
Intravascular Coronary Lithotripsy
Intravascular lithotripsy (IVL) is a promising new method for the treatment of heavily calcified coronary lesions that received a CE mark in May 2017. Coronary lithotripsy is based on the fundamental principles of lithotripsy, a technology that has been used to break up kidney stones for over 30 years. Pulsatile mechanical energy is used to fragment selectively amorphous calcium, sparing soft tissue.
The IVL system (Shockwave Medical) is made up of three components: a battery-powered rechargeable generator capable of producing 3 kV energy and preprogramed to deliver a fixed number of pulses per balloon, a cable connector that links the generator with the catheter and a single-use sterile catheter with a semicompliant balloon and three miniaturised lithotripsy emitters distributed along the length of the balloon.80 These emitters convert electrical energy into transient acoustic pressure pulses (1 pulse/s for a maximum of 80 pulses per catheter). IVL balloons are available in sizes ranging from 2.5 to 4.0 mm, with a unique maximum length of 12 mm. After the lithotripsy balloon is inflated to 405 kPa, pulsatile energy is emitted for 10 seconds from two emitters localised within the balloon (the distal emitter is slightly more central to enhance flexibility, whereas the proximal emitter is located near the proximal end of the balloon); the balloon is then inflated to 608 kPa. These balloons are compatible with 5 and 6 Fr guide catheters but have a rather large crossing profile of 0.043–0.046 inches. Therefore, in some cases a gentle predilatation with a traditional 1.5 mm or 2.0 mm balloon is necessary before IVL balloon inflation.
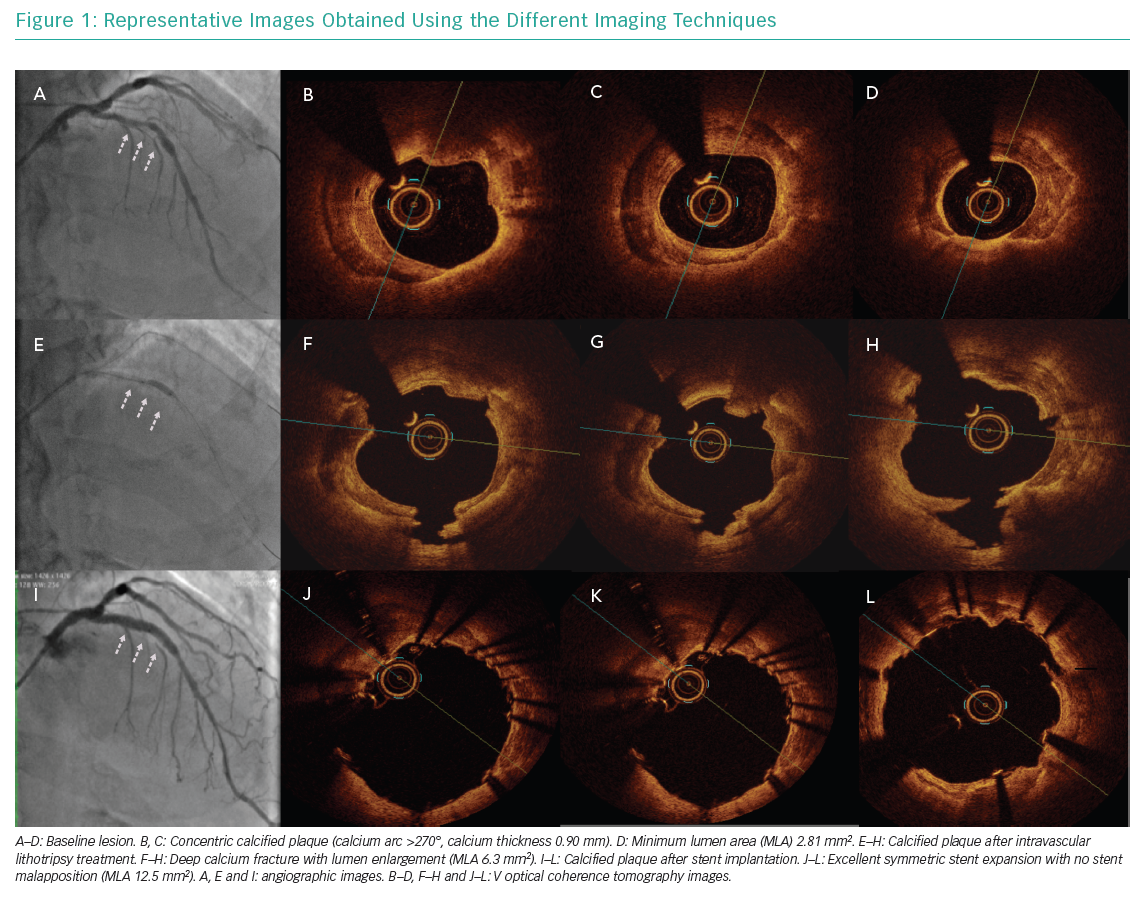
The mechanism of action was described by Ali et al., who used OCT in 31 patients with severe coronary calcified lesions treated with IVL.81 In that study, lumen enlargement and single or multiple calcium fractures were observed after IVL in 43% of patients. The Shockwave Coronary Rx Lithoplasty® Study (Disrupt CAD I), a single-arm feasibility trial, enrolled 60 patients with calcified coronary artery disease treated with IVL across seven countries.82 The primary endpoint (residual diameter stenosis <50% after stent implantation without in-hospital MACE) was achieved in 98.5% of patients (residual stenosis mean ± SD: 13.3 ± 11.6%) and acute luminal gain was 1.7 ± 0.6 mm. Analysing data from that study indicated that the technique appeared safe with no major periprocedural complications (no residual dissections, no perforation, no slow flow or no-reflow).
The Disrupt CAD II trial completed enrolment of 120 patients in 15 hospitals in April 2019.83 Preliminary data indicate successful delivery of the IVL balloon in all patients, with 34% facilitated by predilation. The post-IVL angiographic acute luminal gain was 0.8 ± 0.5 mm and residual stenosis was 29 ± 12%, which further decreased to 12 ± 5% following DES implantation. Residual stenosis <50% was achieved in all patients, with freedom from in-hospital MACE in 94.2% of patients (5.8% of patients had asymptomatic non-Q wave MI without clinical sequelae). Case series (Figure 1) and single-centre registries confirmed the efficacy and safety of this new technique, but no data are currently available from randomised trials comparing IVL with modified balloons or RA.84,85 An Irish retrospective review of 54 patients treated with IVL reported a higher incidence of isolated ventricular capture beats or asynchronous cardiac pacing during shockwave pulse with no clinical events associated with this phenomenon.86
Authors’ Perspectives
There have been substantial technological advancements in the treatment of calcified coronary lesions in recent years, with higher procedural success and lower periprocedural complications due to a combination of more reliable, stronger NC balloons, scoring and cutting balloons, RA and, more recently, OA and intravascular coronary lithotripsy.87 Second-generation DESs and more effective antiplatelet agents decrease thrombosis and restenosis. Despite these encouraging data, in clinical practice all the dedicated devices for the treatment of calcified lesions, and especially RA and OA, are grossly underutilised because of a perceived greater procedural risk in the hands of inexperienced operators and substantial costs.
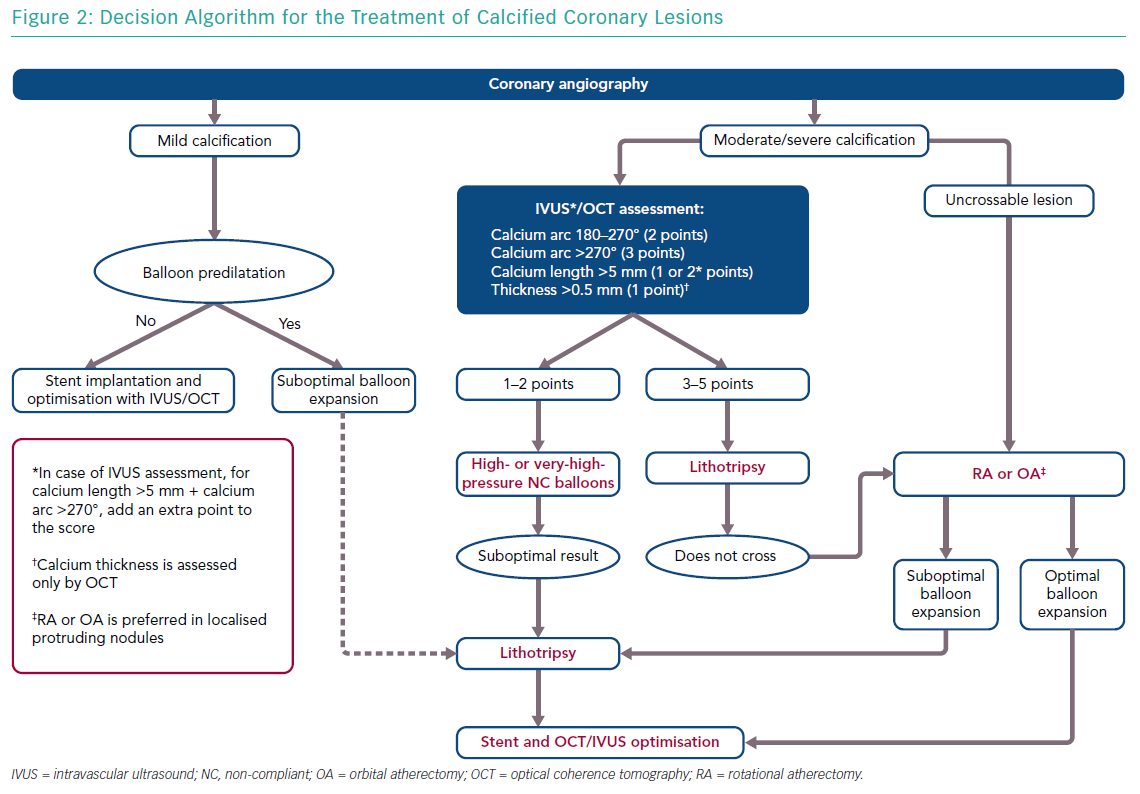
In this context, we believe that IVL has the potential of more widespread adoption because of its efficacy, at least in concentric calcified lesions, safety (with a short learning curve), low periprocedural complications and no risk of coronary perforation. In light of these changes, we propose a new algorithm to approach the treatment of coronary calcified lesions after preliminary characterisation based on multimodal imaging. OCT is certainly the preferred intravascular imaging technique for calcified coronary lesions, but it is not feasible in some cases, such as in moderate to severe renal failure or in severe stenoses. In such cases, IVUS can quantify calcium arc and calcium length, but not calcium depth.
For this reason we have modified the OCT score proposed by Fujino et al.26 to enable the use of OCT with IVUS, adding an adjunctive point for lesions with a calcium arc >270° and calcium length >5 mm because, based on our experience, deeper calcified lesions have concomitantly more concentric calcium and longitudinally extended calcium. In addition, another point has been added for lesions with an arc >270° because, based on the literature, the efficacy of IVL was greater in these lesions. Analysing these parameters, a decisional algorithm was proposed to guide the interventional cardiologist to choose the most appropriate device (Figure 2). In case of unsuccessful or partially successful treatment, more devices can be used in sequence, as reported in a recent case of RotaTripsy treatment (a combination of RA and IVL).88
Conclusion
The problem of heavily calcified coronary lesions will increase in the future because of an aging population and increased rates of diabetes and chronic renal disease. An approach based on multimodal imaging techniques and the use of dedicated devices is key to improve patient outcomes. Further data will be needed to confirm the efficacy of these new, dedicated devices on long-term clinical outcome.