The optimal assessment and management of patients presenting with recent onset chest pain is achieved by acquiring information about the coronary anatomy and physiology, enabling decisions to be made based upon evidence of ischaemia at a patient-level and at lesion-level. There are robust data indicating that the visual assessment of stenosis severity alone does not provide enough information upon which to base decisions about revascularisation.1,2 The addition of an assessment of vessel physiology in the form of indices derived from invasive pressure wire, such as fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR), allows for more accurate identification of lesions causing myocardial ischaemia that facilitates personalised management of coronary disease.3–7 Despite this evidence, which has resulted in international guidelines recommending the routine use of FFR and iFR, the uptake in clinical practice has been inexplicably low.8–12 Potential reasons for this reduced rate of adoption include increased procedural time, concerns about the added procedural risks, technical uncertainty about pressure wire drift or damping, increased costs, the requirement for patient counselling before the procedure and, perhaps most importantly, the need for experienced operators.13
Some of these factors have driven the evolution of invasive physiological methodology. Specifically, concern about preparation time, side-effects, additional costs and availability have led to the development of invasive adenosine free, non-hyperaemic methods in addition to iFR, such as distal coronary pressure to aortic pressure ratio (Pd/Pa), diastolic pressure ratio (dPR/DPR), resting full-cycle ratio (RFR), diastolic hyperaemia-free ratio (DFR) and contrast FFR.14–16 These indices have been shown to correlate closely with the gold standard FFR and iFR, but they all require an intracoronary pressure wire.17,18
In parallel, there have been important advances in methods that derive anatomical and physiological information from computational or mathematical modelling. By applying computational fluid dynamics (CFD) to the raw data from the CT coronary angiogram, FFR can be predicted in all major epicardial vessels to produce FFRCT non-invasively and this technology has been developed by HeartFlow (Redwood City, CA, US). Subsequent to clinical validation and cost efficacy studies, this technique has been approved by the National Institute for Health and Care Excellence (NICE) and is the subject of an Innovation and Technology Payment programme from NHS England to encourage its uptake in clinical practice.19–21 The aim of this technology is to reduce unnecessary invasive coronary angiography in patients presenting with chest pain. The next challenge is how to increase the use of physiological coronary assessment for those patients who do make it to the catheter laboratory.
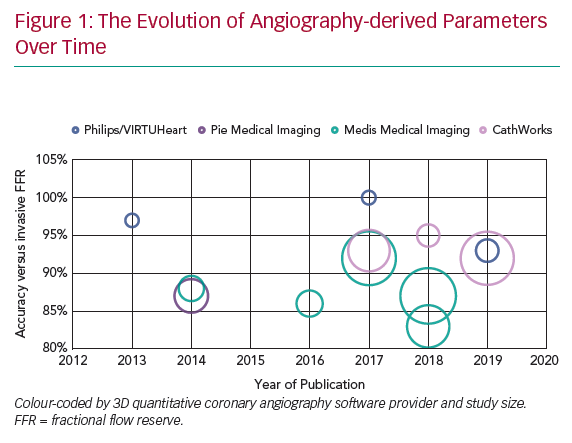
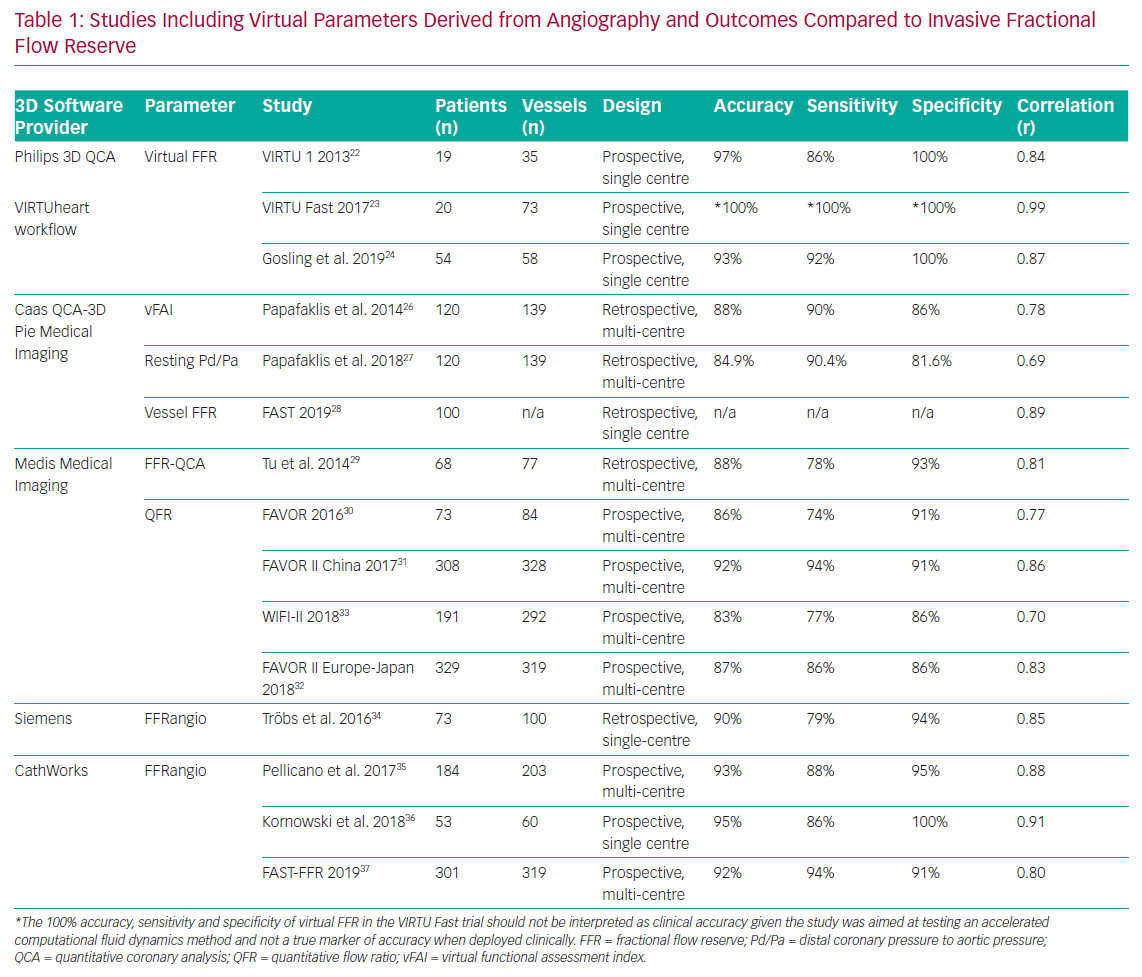
Several research teams have developed systems (Figure 1 and Table 1) that derive physiological data from invasive angiographic images resulting in simulated physiological indices that are surrogates of invasively measured FFR. This review describes the currently available systems offering computed coronary physiology derived from angiography and discusses the potential value of these tools in the clinical assessment of patients.
Angiography-derived Physiology Systems
Virtual Fractional Flow Reserve
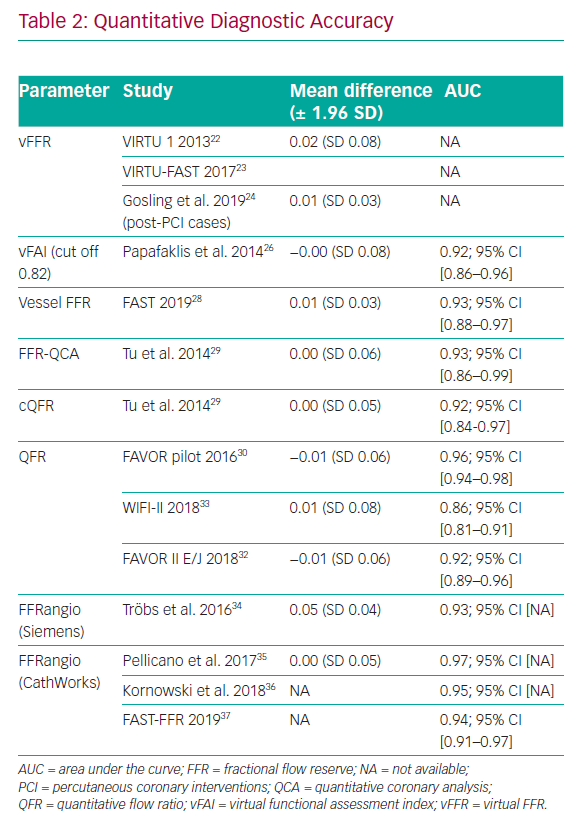
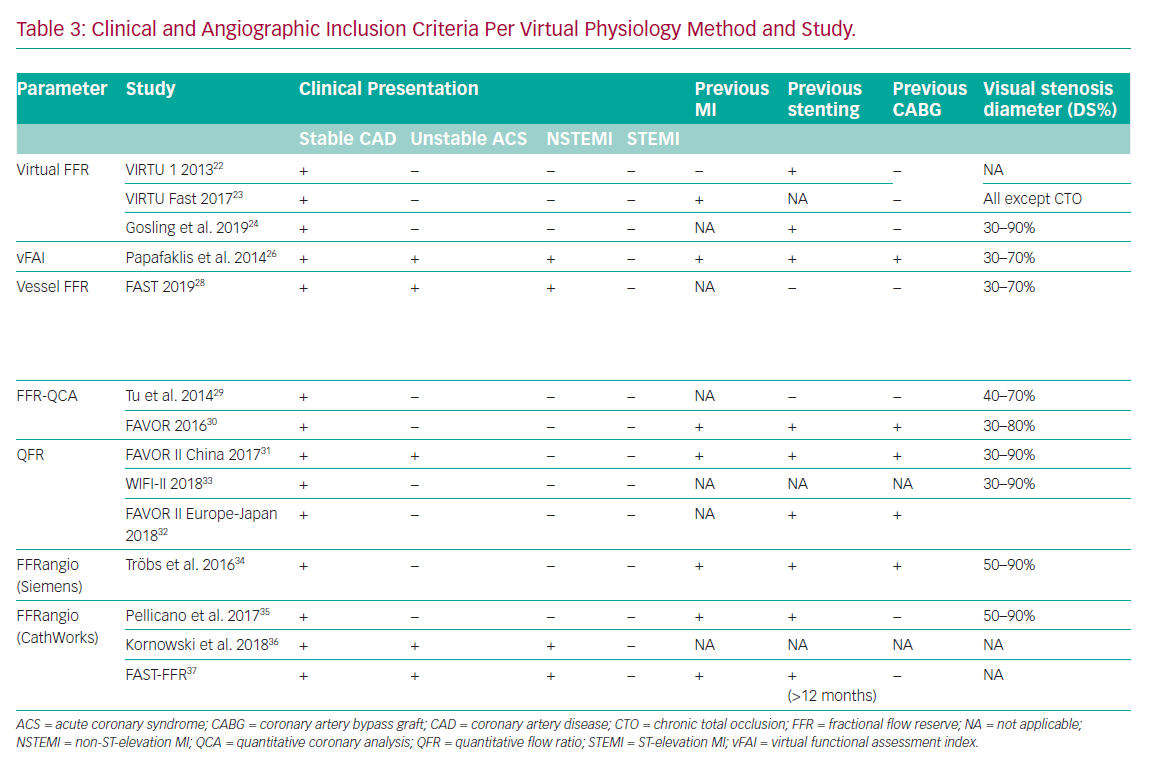
The original computed or virtual FFR (vFFR) model was modelled using the VIRTUheart software devised by a team at University of Sheffield (UK). This used rotational coronary angiography and a Philips 3D workstation to reconstruct the 3D coronary artery anatomy in silico. After reconstruction, mathematical boundary conditions were assigned (representing the physiological conditions which bound the reconstructed 3D domain) at the inlet and outlet. CFD simulation was then performed by Ansys CFX to yield the vFFR result. vFFR showed a diagnostic accuracy of 94% to predict physiological significance in a modest cohort of 19 patients – with 13 of them also having percutaneous coronary intervention (PCI) with post-stenting cases. However, performing the full 3D, time-dependent (or transient) 3D CFD required >24 hours of computing time.22
The Fast Virtual Fractional Flow Reserve Based Upon Steady-State Computational Fluid Dynamics Analysis (VIRTU-Fast) study, from the same University of Sheffield researchers, demonstrated a ‘pseudotransient’ or paired steady-state analysis, that accelerated processing to 189 seconds with <1% error relative to fully transient CFD.23 This >500-fold CFD acceleration method was then incorporated into their proprietary VIRTUheart workflow. The same study included a sensitivity analysis which investigated the sensitivity of vFFR relative to model inputs, that is, markers of stenosis geometry, proximal pressure and microvascular resistance. This analysis demonstrated that accurate vFFR computation was heavily dependent upon the tuning of the boundary condition of the distal vessels – the values used to represent microvascular resistance in an individual case.
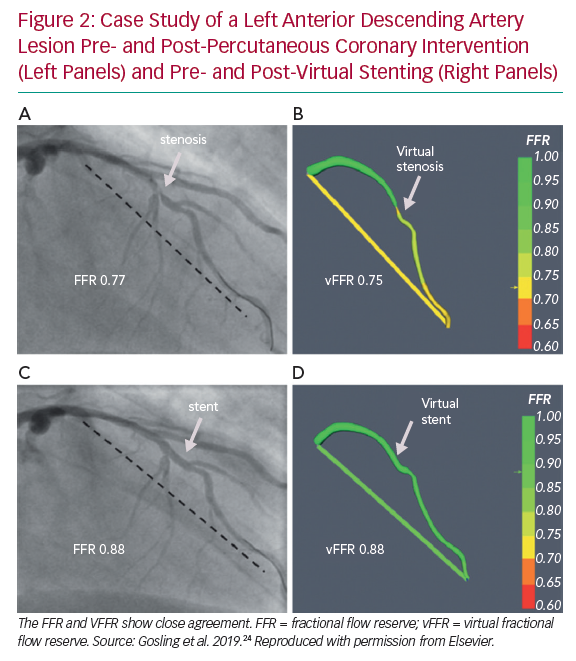
The group now model vFFR using two image projections ≥30° apart from standard multiplanar angiography. CFD computation time was reduced further to a mean of 95 seconds while achieving excellent accuracy, specificity and sensitivity (93%, 92% and 100%, respectively). In addition, a virtual stenting tool enables operators to assess the physiological impact of multiple alternative stenting strategies to identify which approach offers the maximum physiological benefit relative to the length of stent deployed virtually, hence potentially allowing a real-time PCI planner (Figure 2). Results correlated closely with the invasively measured post-PCI FFR (r=0.80).24
There are certain limitations of this technique. First, the studies only included a small number of cases all recruited in elective, stable circumstances (Table 3). Complex coronary anatomies including total occlusion and coronary artery bypass grafts (CABG) were excluded. Second, applying a ‘one size fits all’ approach to tuning the models (i.e. reflecting the microvascular resistance or hyperaemic flow on an individual case basis) results in error in cases where these parameters are different from population-averaged values.25 Hence results may prove to be different in patients with scarring, previous infarcts or left ventricular hypertrophy, for example. However, this is a universal challenge for virtual physiology modelling and highlights the difficult choice of assumptions that need to be made when constraining the mathematics and tuning the boundary conditions.
Virtual Functional Assessment Index and Virtual Distal Coronary to Aortic Pressure Ratio
Papafaklis et al. developed a simplified approach for virtual functional assessment of coronary stenoses from routine angiographic data using the Caas Workstation Quantitative Coronary Analysis (QCA)-3D from Pie Medical Imaging (Maastricht, the Netherlands) to recreate coronary geometry. Further processing involves steady-flow CFD analysis using flow rates of 1 and 3 ml/s, corresponding to the average flow at rest and during hyperaemia. The pressure gradients at these two flow rates were calculated from the difference of the average pressure at the inlet and outlet and a ratio of Pd/Pa was derived. The average computed pressure ratio over this flow range was named the virtual functional assessment index (vFAI). This model was studied retrospectively in a multicentre cohort of 120 patients and 139 vessels. It demonstrated that, for an optimum vFAI value of 0.82, the ability to discriminate ischaemia-producing lesions was very good with the area under the receiver operating characteristic curve (AUC) 92% (95% CI: [86–96%]), and the correlation with invasive FFR was reasonable (r=0.78). Diagnostic accuracy, sensitivity and specificity were 88%, 90% and 86%, respectively.26 The virtual resting Pd/Pa with an optimal cut-off ≤0.94 performed less well when correlated to invasive FFR (r=0.69).27
A limitation of this system is that the processing was 15 minutes per vessel, which is not ideal for the workflow in a typical catheterisation laboratory. In addition, vFAI is entirely a function of the geometry of the stenosis. It cannot be a surrogate for FFR because FFR is a measure of the pressure gradient in the context of patient-specific, microvascular, physiology. It is therefore likely to be less accurate in patients with microvascular disease, scar or increased myocardial mass.
Vessel Fractional Flow Reserve
Using the same Caas Workstation QCA-3D, Masdjedi et al. have proposed a new method for simulating FFR from conventional angiography. The 3D coronary reconstruction is processed semi-automatically based on two angiographic projections. The boundaries were defined as a constant parabolic flow incorporating the measured aortic pressure at the inlet and stress-free (zero pressure) at the outlet approximating the coronary wall as rigid and the blood as Newtonian fluid. The pressure drop is then calculated based on physical laws incorporating viscous resistance and separation loss effects resulting in a parameter named vessel-FFR (commonly given the acronym vFFR, not to be confused with virtual FFR described above, which is computed via a different method). This algorithm was then retrospectively applied to a cohort of 100 patients with an intermediate degree of stenosis in the Fast Assessment of STenosis severity (FAST) study that found a good correlation between the offline simulated vessel FFR and invasive FFR (r=0.89; p<0.001). Bland Altman limits of agreement were impressive at ± 0.03.28
Departing from CFD using Navier-Stokes equations and instead using simplified haemodynamic laws renders much faster analytical results, reported as ‘instantaneous’ by the authors. However, it should be noted that this technique required manual input for the 3D reconstruction and the time that this took was not quantified. In addition, several assumptions are required when the mathematics are simplified. This again highlights the challenges of incorporating patient-specific parameters into the solution, particularly regarding either hyperaemic flow and/or microvascular resistance. Finally, these results were collected retrospectively in a small cohort of selected patients and without involving a core lab. It will be interesting to see if the high levels of diagnostic accuracy and relatively narrow limits of agreement are reproduced in larger multicentre trials when the tool is used by others. Some of these limitations may be addressed in the FAST-II study (NCT03791320), a prospective multicentre observational trial using this technique which was aiming to finish recruiting at the end
of 2019.
Quantitative Flow Ratio
This model was initially based upon two angiography projections during rest and at least one additional projection obtained during hyperaemia which were further processed using QAngio XA 3D prototype by Medis Medical Imaging (Leiden, the Netherlands) to generate the 3D QCA. The steady-state CFD analysis was then performed using the hyperaemic flow velocity derived from thrombolysis in MI (TIMI) frame count at the boundaries. The parameter that resulted, initially named FFRQCA, took 10 minutes per vessel and showed good correlation (r=0.81; p<0.001) with invasive FFR in a cohort of 68 patients and 77 vessels.29 The main drawback of this initial method was the need to use adenosine for hyperaemia. A unique benefit in this approach was that it incorporated a surrogate of patient-specific flow velocity from TIMI frame counting the progress of the contrast through the artery being studied. Other models must assume this value based on less specific markers.
Subsequently, however, this approach was modified such that the flow velocity was frame-counted under resting conditions and an assumption/prediction was then made about what the velocity would be if hyperaemia was induced. Thus, the hyperaemic adenosine model quantitative flow ratio (aQFR) was replaced with the contrast quantitative flow ratio (cQFR). This was validated in the Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve from Diagnostic Coronary Angiography (FAVOR) pilot study involving a cohort of 73 patients and 84 vessels. The cQFR (or simply QFR from here on) showed good accuracy compared with invasive FFR (86%).30
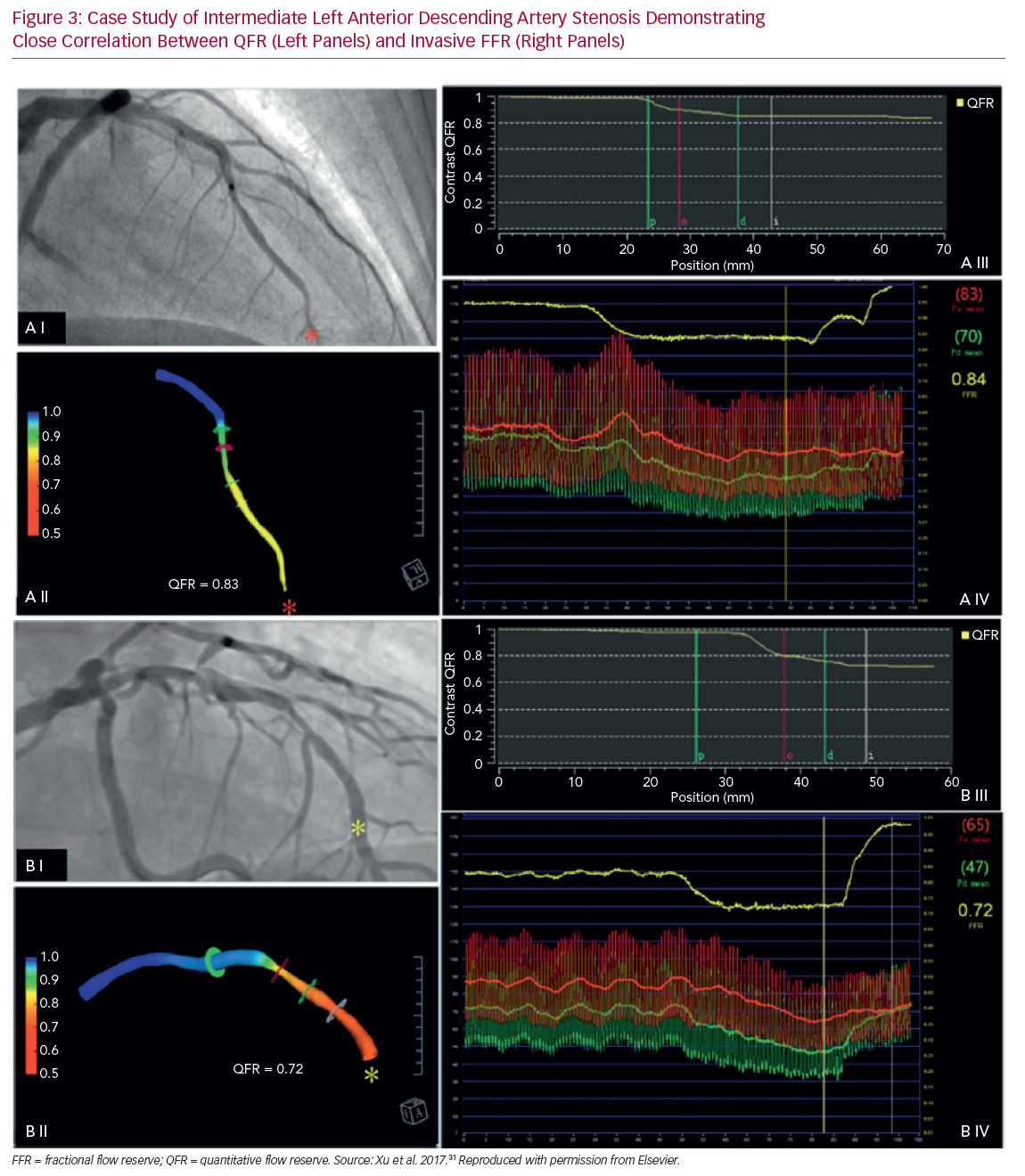
Three further prospective multicentre trials validated the method in Asia and Europe and included more than 800 patients in total (Table 1 and Figure 3).31–33 A major benefit of computed FFR is that it may reduce procedure time relative to invasive FFR with QFR processing time being 5 minutes compared with measuring invasive FFR, which took 7 minutes.31 For speed and computational simplicity, CFD equations were replaced with equations based upon the laws of Bernoulli and Poiseuille. QFR was derived from relatively few parameters such as vessel geometry, assumptions on flow through a stenosis and vessel tapering in relation to side branch, thus generating a calculation of function based primarily upon anatomy. Not all vessels are currently suitable for this approach with a 6–31% reported attrition rate.32,33
FFRangio (Siemens)
Another technique, termed FFRangio, was developed in collaboration with Siemens Healthcare GmbH (Frochheim, Germany), which provided the 3D reconstruction software and the CFD modelling prototype. The boundaries of the 3D model were personalised by using the blood pressure measured at the tip of the catheter and heart rate. FFRangio required only 40 seconds per vessel mean calculation time and, using the same 0.80 cut-off, reached a 90% agreement with invasive FFR in deciding whether a lesion was significant or not. The Bland Altman limits of agreement were excellent at ±0.04. The limitations of this technique so far are similar to those of other systems. Validation data are based on a limited number of patients included in the retrospective study (73 patients, 100 vessels).34 Additional drawbacks were loss of data from the final analysis due to motion artefacts, elements of the workflow requiring manual input and estimating microvascular resistance. To date there have been no further published trials using Siemens software.
FFRangio (CathWorks)
FFRangio is now a CathWorks Ltd (Kfar Saba, Israel) registered trademark which has developed a proprietary method for computing the ’functional angiogram’ of the entire coronary tree. The basis is similar to other methods in that it constructs the 3D coronary model from single plane angiographic projections, initially requiring at least three, rather than two, projections. This model applies a serial resistance model based purely on the anatomical features of lesions (such as length and diameter) to calculate resistance, while neglecting entrance effects and rheology particularities. Pellicano et al. validated the technique in a prospective, blinded, multicentre trial including 184 patients using the offline version and reporting a 93% accuracy compared with invasive FFR.35
Kornowski et al. prospectively tested FFRangio online in a cohort of 53 patients and 60 vessels and obtained excellent concordance with FFR (AUC 0.95) within 10 minutes.36 Recently, Fearon et al. have published the results of the FAST-FFR trial, which is a prospective, multicentre international trial including 301 patients and 319 vessels employing an updated version of the proprietary technology from CathWorks, requiring only two angiographic projections for the 3D reconstruction. In the per vessel analysis, the results were encouraging, with sensitivity and specificity at 94% and 91%, respectively, and an overall accuracy at 92% using invasive pressure wire as the reference. The 99% success rate of the device was particularly impressive.37 The main advantage of this model lies in generating a complete and accurate 3D reconstruction, including the branches (down to 0.5mm), coupled with a short processing time. However, once again, there are considerable assumptions in the mathematical solution that may compromise the model’s ability to accurately characterise haemodynamic flow and translesional pressure dynamics.
Potential Advantages of Angiography-derived Physiology
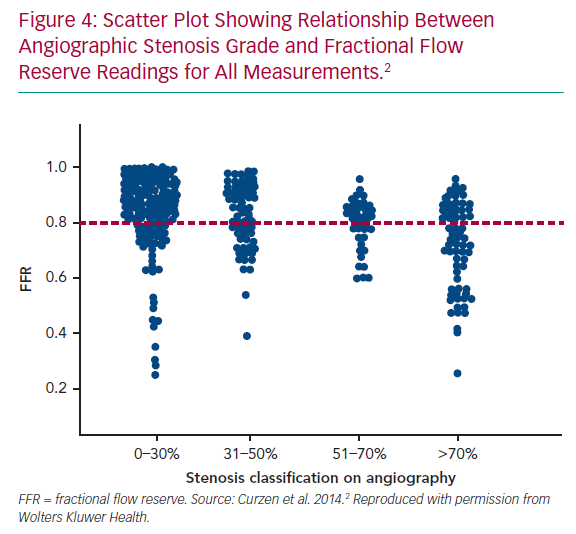
The ability to assess the physiology of all epicardial coronary branches and potentially intervenable lesions directly from the invasive angiogram could be a game-changer for personalised patient management. Given the extensive data that support the clinical value of knowing the anatomical atheroma burden, but also lesion-level ischaemic potential, deriving functional data from the anatomy, without needing to use an invasive pressure wire at all, let alone in multiple vessels, could facilitate achieving this management paradigm. While different systems are competing for this role, they all have the major advantage of not requiring a pressure wire or adenosine. This resolves the key limitations of invasive FFR: the cost and time associated with pressure wire use and adenosine administration, as well as the required technical expertise and potential complications when passing an intracoronary wire. Furthermore, these models offer the potential for global assessment of all major vessels in a single analysis thereby providing a more comprehensive assessment and reducing the operator bias that affects use of invasive pressure wire. Typically, operators interrogate lesions with 50–70% stenosis, but not those with 30–50% or 70–90% stenosis, despite a wealth of data reporting that these categories also comprise anatomy-physiology discordant cases (Figure 4).38
The 3D reconstruction of the coronary arteries is based upon two 2D projections acquired during routine coronary angiography.37 Computation appears to be operator-independent and highly reproducible, with accuracies between 83 and 95% compared with invasive FFR (Table 1).9,28,29 Furthermore, a meta-analysis including 13 studies with most, but not all, of the methods described above, and a total of 1,842 vessels demonstrated a good pooled diagnostic accuracy when compared with invasive FFR using the 0.8 cut-off (AUC 0.84; 95% CI [0.66–0.94]). The pooled sensitivity for detecting a significant coronary stenosis was 89% (95% CI [83–94%]) and the pooled specificity was 90% (95% CI [88–92%]), without any significant difference between calculation methods (CFD versus mathematical), software or analysis type (offline versus online).39 However, diagnostic accuracy is a function of model accuracy as well as the clinical case mix included in a particular study. For the same virtual physiology model, diagnostic accuracy will appear high in studies with comparatively fewer patients around the 0.80 threshold, but far worse if the opposite is true. A better statistical test to characterise the accuracy of the model is a Bland Altman plot with ±95% limits of agreement (± 1.96 SD). From this perspective all methods are comparable with mean differences between -0.01 and +0.05 and SD between ± 0.03 and ± 0.08 (Table 2).
Processing time for all models has been reduced so they could be integrated into the workflow of a typical catheterisation laboratory. Finally, some of these systems simulate and predict the physiological outcome of alternative PCI strategies in real time, enabling the identification and selection of the optimal revascularisation approach.24
Limitations of Angiography-derived Physiology
Methodologically, key challenges remain in adequately representing patient-specific physiology. Specifically, this means tuning models with artery-specific data that reflect microvascular resistance or hyperaemic flow. Both parameters vary in health and disease and are difficult to measure invasively, let alone predict non-invasively. Without adequate physiological ‘tuning’, these models are little more than a sophisticated QCA, dependent only on stenosis geometry, ignoring the very things that make FFR superior to diagnostic angiography. The whole point of FFR is that it reports the significance of a lesion, and thus the potential benefit of PCI, in the context of the patient’s microvascular resistance. Failure to reflect this in computed FFR would represent a retrograde step in clinical assessment, not an advance.
A further limitation is that, despite impressive diagnostic accuracy, the confidence intervals for these tools remain wide for borderline FFR cases. In addition, all these methods rely upon good quality angiography, in which clear, unobstructed, high contrast images with minimal overlap, panning or excessive magnification (cutting off proximal or distal vessel) are recorded. Furthermore, some need an ECG signal to identify diastole. An invasive pressure wire examination on the other hand, requires less angiographic precision. Also, the segmentation step when the downloaded angiographic images are reconstructed into a 3D in silico anatomical model, requires considerable practice, time and skill, because manual corrections are often required.40
In terms of validation, all methods have been compared only with a dichotomised application of invasive FFR, with its inherent shortcomings when considering there is a continuum of risk associated with reduced coronary flow and there is a lack of prospective outcome studies (Table 1). Furthermore, studies include mostly stable angina and intermediate lesions within 30–90% (Table 3), excluding chronic total occlusion, proximal left main and right coronary artery and side branch stenosis.31,33 A number of studies have included patients with previous MI or revascularisation and others have included acute coronary syndromes.13,26,27,37 Work is under way to investigate the role of QFR in assessing non-culprit lesions in ST-elevation MI (STEMI) patients (Non Culprit Functional Evaluation With 3D Angio QFR in STEMI PCI Procedure [NCT02998853]), which has assumed increased importance since the recently reported Complete Versus Culprit-Only Revascularization Strategies to Treat Multivessel Disease After Early PCI for STEMI (COMPLETE) trial.41
Some of these techniques have been validated using higher than standard temporal resolution (15–30 frames/s), thus increasing the radiation exposure for patients and operators.29,34,35 Not all angiograms are suitable for computational analysis because this requires optimal coronary opacification, a lack of overlapping vessels and excellent views of the lesion in at least two projections. It is estimated that up to one-third of standard angiograms may be unsuitable, although operators may improve their technique to meet modelling demands in selected patients.33
Regulatory Approval
vFFR (CAAS workstation) was the first method to translate into practice after receiving US Food and Drug Administration (FDA) market clearance in March 2018 followed by CE mark in Europe. FFRangio was granted FDA market clearance in December 2018. It has a CE mark in Europe and Medical Devices and Accessories (AMAR) approval in Israel and Pharmaceuticals and Medical Devices Agency (PMDA) approval in Japan. QAngio XA 3D/QFR was CE marked in Europe as a class IIa device in 2017. It was reviewed by NICE in the Medtech innovation briefing, in which the specialist panel saw this technology as “potentially replacing FFR and iFR… could also reduce other functional tests… and FFRCT”.42 Formal guidance is expected at the end of 2020.43 In May 2019, the FDA granted QAngio XA 3D/QFR market clearance.
Conclusion
Significant steps have been made in developing angiography-derived coronary physiology tools which compare favourably to the gold standard invasive FFR. Three methods are already available on the market and others have shown encouraging prospects. Although there are still methodological challenges to be negotiated, all techniques allow the assessment of the functional significance of stenoses in all epicardial coronary vessels without any additional risk. Furthermore, they offer the possibility of virtual pull-back at multiple levels in the coronary tree, thus providing a more comprehensive assessment at lesion-specific, as well as vessel-specific level. This could represent the dawn of a new era in which the gold standard invasive FFR is replaced by computed algorithms that simulate physiology comprehensively throughout the epicardial coronary tree in real time in the catheterisation laboratory. Such an approach could prove particularly useful for non-PCI centres that undertake diagnostic angiography. Furthermore, some systems may allow real-time stenting procedure planning facilitating bespoke treatment algorithms for patients. However, more clinical data, including randomised trials, demonstrating prognostic clinical benefit are required before any of these methods make their way into routine practice.