Coronary artery bifurcation lesions are treated in 15–20 % of percutaneous coronary intervention (PCI) procedures and are still plagued by worse outcomes.1 This is in spite of recent significant advancements in stent technology in general and in bifurcation stenting techniques in particular. Conventional angiography provides only limited information about bifurcation anatomy, plaque distribution and stent apposition and expansion.
As for all complex lesions, intravascular ultrasound (IVUS) has shown advantages over conventional angiographic guidance. Optical coherence tomography (OCT) has a resolution 10 times higher than that of IVUS and is able to provide valuable information at each step of PCI. This includes the measurement of coronary lumen in the mother vessel and daughter branches, the detection of plaque location, the planning of the most convenient stenting approach and the evaluation of stent expansion and strut apposition.2
Optical Coherence Tomography Imaging and Analysis
OCT examination of bifurcation lesions should be performed in both the main vessel (MV) and the side branch (SB). Predilatation is often required to allow lesion crossing and effective blood clearance during the examination.
Automatic pullbacks are performed (usually at 20 mm/s) during contrast injection at a rate of 3 ml/s (right coronary) to 4–5 ml/s (left coronary) – ideally using a power injector. Full replacement of blood with clear crystalloid contrast is fundamental to clear the blood from the vessel to obtain diagnostic images, which can be challenging in large vessels. Optimal sitting of the guiding catheter should be checked before starting the pullback.
A 6 Fr catheter is normally sufficient for optimal imaging, allowing the use of 6 Fr guiding catheters as standard. The OCT catheter is inserted distal to the lesion or stent to be examined, and the pullback continued until either the guiding catheter is reached or the maximal pullback length is completed.
A longitudinal cross section of the entire vessel length is automatically generated and the cross sections of interest can be scrolled. Moreover, the real-time co-registration of OCT images with angiography facilitates combined angiographic and intracoronary high-resolution imaging of coronary arteries directly at the catheter laboratory, where the exact localisation of the acquired OCT frame can be displayed side-by-side on the angiogram. This allows a more precise identification of the carina and side-branches. Because of the clear interface between the lumen and wall, current software provides automatic detection of the lumen area and the percentage of area of stenosis, comparing user-defined reference and stenotic segments. Most OCT users value the possibility to detect in approximately 70 % of distal reference segments the media and obtain a manual measurement of the media-to-media diameter.
The recent Optical Coherence Tomography Compared With Intravascular Ultrasound And Angiography To Guide Coronary Stent Implantation: A Multicenter Randomized Trial In Percutaneous Coronary Intervention (ILUMIEN III: OPTIMIZE PCI) study obtained superior results compared with the previous OCT/IVUS head-to-head comparisons.3 The study employed a specific OCT-guided stent optimisation algorithm based on measurement of the external elastic lamina in the proximal and distal reference segments, designed to achieve larger stent dimensions and more complete lesion coverage. However, the low-depth penetration of light through lipid-rich plaque often results in an inability of OCT to visualise the true vessel size (delineated by the external elastic lamina) at the lesion site, which is why the use of the media-to-media diameter is widespread.
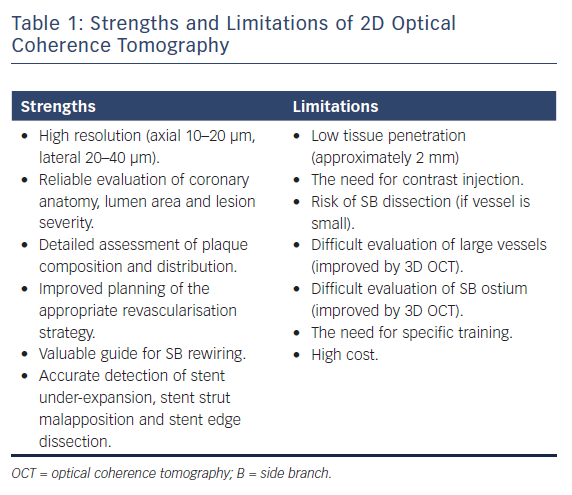
While the main goal of the OCT examination is the identification of proximal and distal reference in the main vessel and the length of the lesion, OCT examination of the SB may be helpful if there are doubts about plaque burden both in terms of severity and length, which are the two main determinants of the need for a two-stent technique. Because each OCT round requires 10–15 ml of contrast, in general the assessment should be performed once before stenting and after optimisation of the stent results based on the diameter measurements obtained with the pre-stent OCT. The repetition of the OCT pullback in the SB after stenting can be avoided when the SB is small or only a provisional approach with no SB stent has been used. On the contrary, in the case of two-stent implantation, the evaluation of SB ostium is of particular importance, often difficult with angiography or OCT examination of the MV alone. 3D OCT reconstruction can, in part, overcome some of the limitations of 2D OCT, allowing a better view of SB ostium and with immediate detection and colour coding of the malapposed struts.4 The strengths and limitations of 2D OCT are summarised in Table 1.
Optical Coherence Tomography-guided Decision Making
Coronary Bifurcation Anatomy, Lesion Severity and Choice of Revascularisation Strategy
OCT provides an accurate evaluation of all the segments of the vessel involved in the bifurcation, including the proximal and distal MV and the SB, with the difficulties related to the assessment of SB ostium discussed in the previous section.
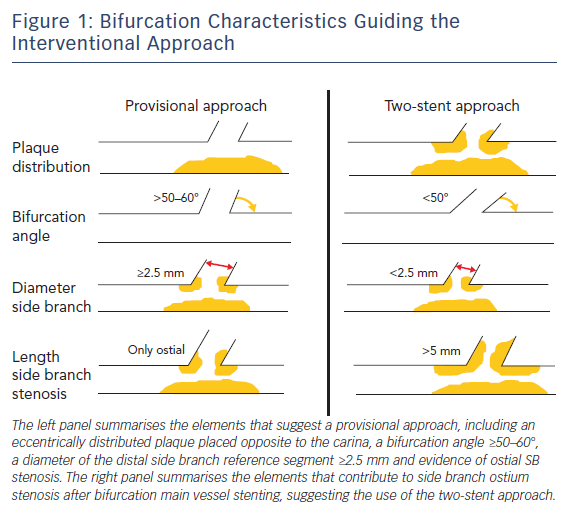
Careful evaluation of bifurcation anatomy and plaque distribution is of great importance because it influences the main decision in the revascularisation strategy: is a single stent likely to maintain good flow in the side-branch as well (provisional single stent) or should a two stent strategy should be the preferred approach from the outset? OCT can identify plaque severity in the main vessel and, when this is distributed eccentrically opposite to the carina, the chances of SB occlusion are small.
The other four elements important for the decision (SB ostial stenosis, bifurcation angle, length of the proximal SB disease, diameter of the distal SB reference segment) can be explored only in part from a MV OCT run. However, in general, the combination of OCT and angiographic information are sufficient for an informed decision (Figure 1). Highly significant correlation between the measurements of coronary lumen performed by OCT and IVUS has been reported, with slight, predictable, overestimation of lumen areas by IVUS.5,6
Watanabe et al.demonstrated that a bifurcation angle <50 % and a length from the proximal branching point to the carina tip of <1.70 mm had a significant prognostic power in predicting SB obstruction after MV stenting.7 Furthermore, high lipid content in the MV lesion and contralateral location of lipid in the bifurcation area – particularly in the proximal wall opposite to the flow divider that is the area with a higher risk of developing vulnerable plaques and vessel rupture – have been shown to contribute to SB ostium stenosis after MV provisional stenting.8 In this context, OCT is able to provide important information about plaque composition and vulnerability compared with IVUS and angiography, being able to effectively detect lipid content of the plaque.
This should be taken into account by the operator when choosing between a single- versus two-stent revascularisation strategy. Indeed, when the risk of SB closure is low, a provisional SB stent implantation strategy should be considered the standard approach for treatment of bifurcation lesions.9 However, when the aforementioned parameters suggest a high risk of SB ostium stenosis or when an ostial stenosis of ≥50 % extending >2.5 mm from the carina is detected, a two-stent technique should be preferred.10,11
Moreover, OCT detects calcium effectively, and can provide information about its thickness and area, along with identifying plaques associated with poor stent expansion that should prompt consideration for pre-stent lesion preparation with techniques such as rotational atherectomy, orbital atherectomy, or cutting or scoring balloons.12
Stent Choice, Implantation and Side Branch Rewiring
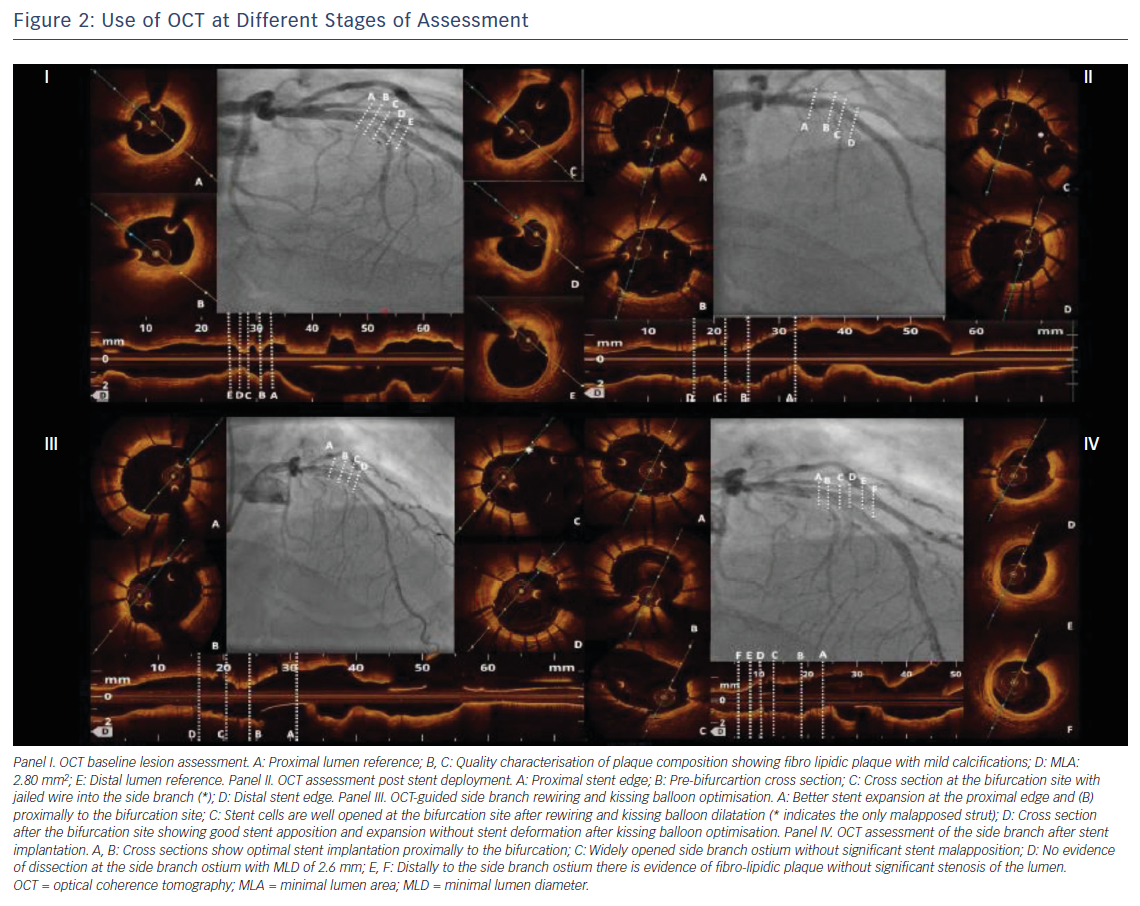
Selecting the correct stent size is particularly relevant in bifurcation lesions because stent oversizing in the MV can determine SB distortion and narrowing because of carina shifting.7 Stents should be sized according to the distal MV reference diameter (Figure 2) and permanent drug-eluting stents should be the stents of choice.10,13 As mentioned, OCT provides reliable measurement of the coronary lumen, improving the selection of the correct stent size, as well as the correct balloon size for the proximal MV stent dilatation, considered a standard step in bifurcation treatment.10
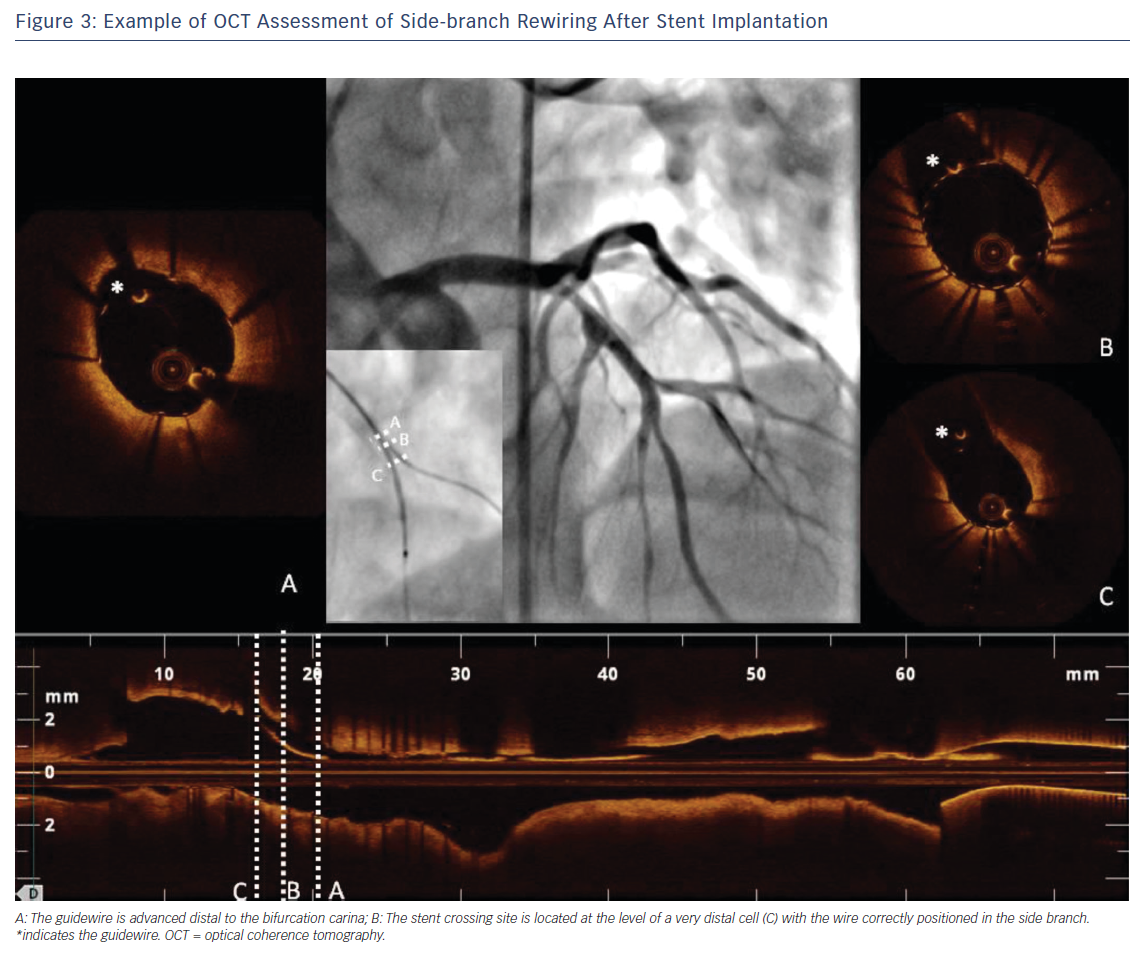
One of the most unique roles played by OCT in the treatment of bifurcation lesions is to guide and optimise SB rewiring. Indeed, wire recrossing to the SB is necessary when a two-stent strategy is chosen or when there is an impaired SB flow after stenting the MV – frequently this step of the procedure can prove challenging. OCT allows a careful evaluation of wire positions after stent rewiring to ensure optimal SB recrossing and to exclude accidental rewiring outside the stent (Figure 3). It has been widely demonstrated that a distal stent cell for recrossing reduces the extent of the metallic carina and favours appropriate stent expansion and stent strut apposition at the ostium of the SB.14,15 In this context, 3D-OCT provides more valuable information than 2D. It allows improved assessment of the SB ostium and rewiring position into the SB and a good detection of the configuration of overhanging stent in front of the SB ostium, reducing the incidence of incomplete stent apposition compared with 2D.4,15,16
OCT has also been used to evaluate the effectiveness of new devices dedicated to bifurcation lesions. The efficacy of the Tryton dedicated side branch stent (Tryton Medical) was tested in small populations17,18 then more widely in the Prospective, Single Blind, Randomized Controlled Study To Evaluate The Safety & Effectiveness Of The Tryton Side Branch Stent Used With DES in the Treatment of De Novo Bifurcation Lesions in the MB & SB in Native Coronaries (TRYTON) trial,19 which failed to demonstrate superiority of this new stent above conventional provisional stenting. Similarly, OCT was used to evaluate the STENTYS stent (STENTYS SA), a provisional, self-expanding nitinol drug-eluting or bare-metal stent reported to have a good success rate along with a low rate of major adverse cardiac events.20
Evaluation of Stent Implantation After Percutaneous Coronary Intervention
After the implantation of one or more stents in the bifurcation, OCT is definitely the technique of choice for the assessment of the final treatment results (Figure 2). Indeed, stent under-expansion, stent strut malapposition, and stent-edge dissection are particularly common in bifurcation lesions and, while often missed by angiography,21 can be accurately detected with the high-resolution images provided by OCT. These findings seem to be related with an increased stent thrombosis and stent restenosis risk.21
Stent under-expansion, defined as an in-stent minimum lumen area <70 % of the average reference lumen area22 or as a minimal stent area of the proximal and/or distal segment <90 % of the proximal and/or distal reference lumen area respectively23, has been correlated with a increased risk of all-cause mortality, myocardial infarction and target lesion revascularisation in several trials that used OCT for the assessment of PCI results. These include the Clinical Impact Of OCT Findings During PCI II (CLI-OPCI II)22 and the ILUMIEN III: OPTIMIZE PCI3 studies. The same trials reported a high incidence of stent strut malapposition and stent edge dissection,3,22 although the clinical importance of these findings is debated.3,21–23 However, evidence of suboptimal stent implantation provided by OCT can prompt the interventionalist to improve the stent placement, guiding the choice of the proper balloon size for post-dilatation and reducing the risk of poor outcomes for the patient.
Conclusion
OCT is a fundamental tool in the interventional cardiologist’s arsenal for the treatment of complex coronary lesions such as bifurcation lesions. Indeed, OCT provides valuable additional information compared with angiography and IVUS that can guide the operator at each step of the PCI – from the planning of the appropriate revascularisation strategy to the assessment of final results.