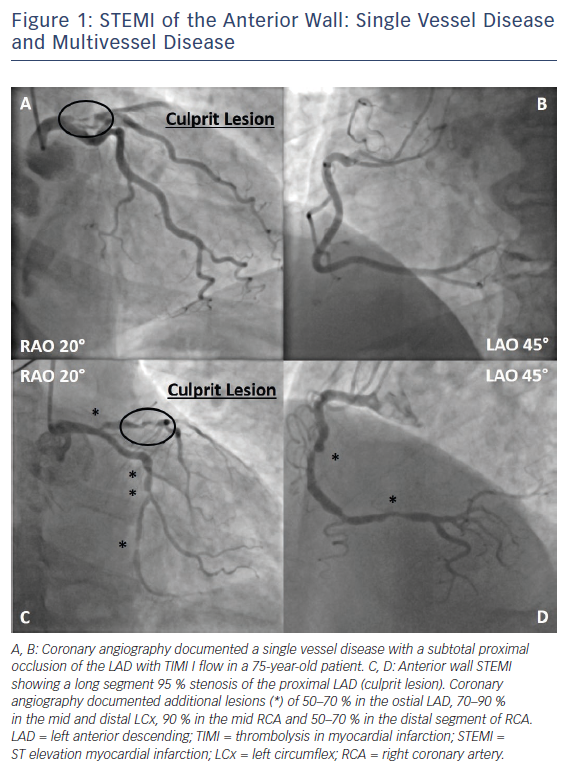
For ST-segment elevation myocardial infarction (STEMI), there is currently no doubt that primary percutaneous coronary intervention (PPCI) of the infarct related artery (IRA) is the preferred reperfusion strategy.1 In about 50 % of cases, STEMI is associated with multivessel coronary artery disease (MVCAD), defined as a ≥50 % stenosis in at least one non-infarct related epicardial coronary artery (N-IRA, Figure 1).2,3 Like many other factors, the number of diseased coronary arteries (‘disease burden’) influences mortality in STEMI patients, thereby doubling the risk of death in the short (30 days, 4.0 % versus 1.9 %) and long term (1 year, 7.0 % versus 3.0 %) in patients with MVCAD in a retrospective pooled analysis of eight randomised STEMI trials.3 Observational and randomised clinical trials so far showed conflicting results regarding the benefit of complete revascularisation, raising the question whether there is enough evidence to change current clinical practice and guidelines.
Complete Versus Culprit Only Revascularisation
Small trials performing intravascular ultrasound (IVUS) have shown differences in plaque characteristics between patients with an acute coronary syndrome (ACS) and stable coronary artery disease (CAD). In ACS, N-IRAs tend to have more high risk features like diffuse CAD and less calcified plaques with thin-cap fibroatheromas and a higher percentage of lipid core, which makes them prone to rupture.4,5 The rationale for complete revascularisation in patients with myocardial infarction (MI) is therefore to reduce the global ischaemic burden and to prevent further cardiovascular events. On the other hand, one might argue that complete revascularisation leads to more procedures, with the associated risks of complications like stent thrombosis, contrast-induced nephropathy (CIN) or stroke, and moreover higher costs.
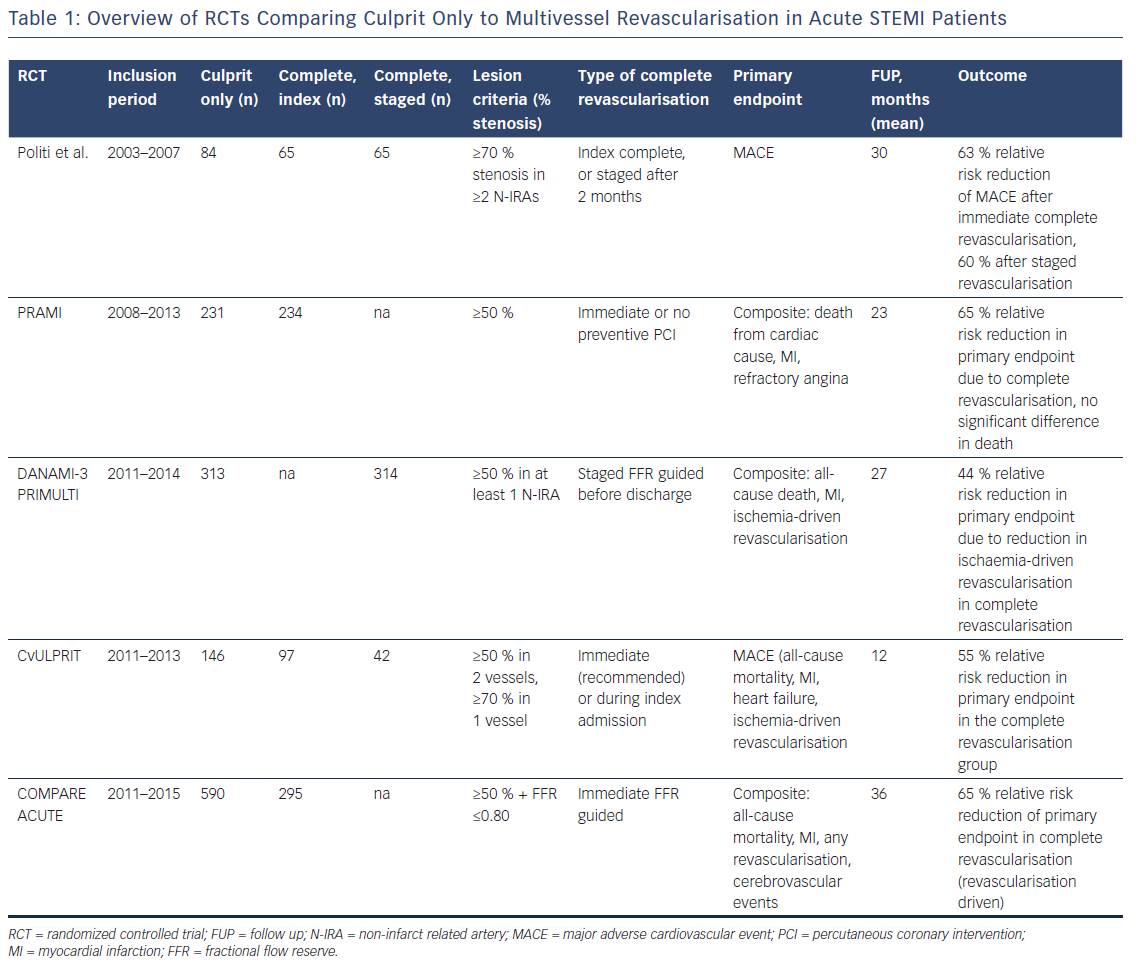
The European Society of Cardiology (ESC) guidelines on Myocardial Revascularisation 2014 recommended PPCI for the culprit vessel, but revascularisation of additional lesions only in the case of cardiogenic shock.2 These recommendations were mainly driven by the results of observational trials showing increased in-hospital mortality, CIN and major adverse cardiovascular events (MACE) in case of multivessel PCI (MVPCI).6–8 The Hepacoat for Culprit or Multivessel Stenting for Acute Myocardial Infarction (HELP AMI) study, was one of the first randomised controlled trials (RCTs) to show that MVPCI was safe without any economic disadvantage.9 More recent RCTs showing that complete revascularisation might be beneficial under certain circumstances led to a change in recommendations in the 2017 STEMI guidelines from the former III to a IIa A recommendation (Table 1).1
In the single blind, randomised Preventive Angioplasty in Acute Myocardial Infarction (PRAMI) trial, a total of 465 patients were evaluated, comparing culprit vessel only PCI (n=231) and immediate MVPCI (n=234) in STEMI. Recruitment was stopped prematurely by the data and safety monitoring board because of highly significant between group differences in favour of preventive PCI.10 The primary composite endpoint, consisting of death from cardiac cause or non-fatal MI or refractory angina, was significantly reduced after MVPCI after a mean follow up (FUP) time of 23 months (HR: 0.35, 95 % CI: 0.21–0.58, p<0.001, 21 versus 53 primary outcomes). This absolute risk reduction of 14 % was evident within 6 months and maintained thereafter. The reduction in risk was similar for the individual secondary endpoints (death from cardiac cause, non-fatal MI, refractory angina, and repeat revascularisation). Of these components, only death from cardiac cause was not significantly different (HR: 0.34, 95 % CI: 0.11–1.08, p=0.07). There was no difference concerning all-cause mortality between the two groups (HR 1.10, 95 % CI 0.38–3.18, p=0.86). Procedure time, fluoroscopy dose and contrast volume increased in these patients, thereby not affecting complications like procedure-related stroke, bleeding and CIN. However, this trial was not designed to address the question of immediate versus staged preventive PCI in STEMI multivessel disease.10
In The Third Danish Study of Optimal Acute Treatment of Patients with STEMI: Primary PCI in Multivessel Disease (DANAMI-3 PRIMULTI), 627 STEMI patients with MVCD were randomly assigned to undergo PPCI of the culprit vessel only (n=313) or staged complete fractional flow reserve (FFR) guided PCI (n=314) before discharge. A FFR value ≤0.80 was considered haemodynamically significant and complete revascularisation was performed a median of 2 days after initial PCI.11 After a median FUP of 27 months the primary combined endpoint of all-cause mortality, re-infarction and ischaemia-driven revascularisation was significantly reduced in the staged FFR-guided complete revascularisation group (HR: 0.56, 95 % CI: 0.38–0.83, 0=0.004). This was mainly driven by a 69 % reduction of repeat revascularisation of the N-IRA (HR: 0.31, 95 % CI: 0.18–0.53, p<0.0001). This effect was more pronounced in young men with anterior MI. However, these subgroups were too small to draw firm conclusions. There was no difference concerning all-cause mortality and non-fatal re-infarction. Furthermore, there were no significant differences of procedure-related complications (MI, bleeding requiring transfusion, stroke or CIN). This trial failed to show differences in hard clinical endpoints like mortality and re-infarction because of a lack of power and like PRAMI, it did not address the question of optimal timing of preventive multivessel PCI.11
Another RCT focusing on preventive MVPCI in STEMI is the Complete versus Lesion-only Primary PCI (CvLPRIT) trial, including 306 patients for IRA PCI only (n=146) or complete revascularisation (n=150) during hospital stay, either during PPCI (recommended, 64 %) or staged before discharge. The primary outcome of MACE (all-cause mortality, recurrent MI, heart failure, ischaemia-driven revascularisation) was significantly reduced in favour of preventive PCI (10 % versus 21.2 %, HR 0.45, 95 % CI 0.24–0.84, p=0.009), leading to a 55 % relative risk reduction in the primary endpoint.12 The individual components of the primary outcome were lower in the complete revascularisation arm, although not statistically significant. Again, Kaplan–Meier curves showed early divergence, with a continuing separation of groups during FUP. There were no differences in the occurrence of serious adverse events between the two groups. Similar to PRAMI and DANAMI-3 PRIMULTI, this trial was not powered to show significant differences in hard clinical endpoints. Furthermore, as almost two thirds of patients were revascularised during initial PCI, timing of preventive revascularisations remains unclear. Like in PRAMI, the CvLPRIT trial did not evaluate the role of FFR for MVPCI.12
Finally, the most recent RCT to address this issue was the Comparison Between FFR Guided Revascularization Versus Conventional Strategy in Acute STEMI Patients with MVD (COMPARE-ACUTE) trial, which enrolled 885 patients. Patients were randomly assigned in a 2:1 fashion to receive either FFR-guided complete revascularisation (n=295) or culprit-only revascularisation (n=590).13 Complete revascularisation was performed in 83.4 % of patients during PPCI in lesions with FFR ≤0.80. The primary endpoint was a composite of all-cause mortality, non-fatal MI, any revascularisation, and cerebrovascular events (MACCE) at 12 months. In COMPARE-ACUTE, MACCE was significantly reduced in preventive FFR guided PCI (23 versus 121 patients, HR: 0.35, 95 %: CI 0.22–0.55, p<0.001), an effect which was mainly driven by a decreased need for revascularisation (HR 0.32, 95 % CI 0.20–0.54, p<0.001). The other components of the primary endpoint did not differ significantly. As there were no significant differences in bleeding, cerebrovascular events and stent thrombosis, this study showed that FFR could be safely performed in PPCI. However, also this trial was not powered to show differences in hard clinical end points.13
Timing of Revascularisation
So far, four different strategies of revascularisation are possible for treatment of MVCD in STEMI: (1) complete revascularisation at the index procedure; (2) complete revascularisation as a staged procedure before discharge; (3) complete revascularisation as a staged procedure after discharge but within a few weeks (not symptom driven); and (4) culprit vessel only PCI. Of the aforementioned RCTs, none included both staged and immediate complete revascularisation and analysed them separately. Politi et al. conducted a small RCT earlier, randomising 214 patients to IRA PCI only (n=81), complete revascularisation during index procedure (n=65), or staged 2 months after index procedure (n=65).14 Patients undergoing staged or immediate procedures had a 63 % (p=0.003) and a 60 % (p=0.002) risk reduction of MACE after a median FUP of 2.5 years, mainly driven by a lower incidence of in-hospital death, re-PCI and re-hospitalisation. There was no significant difference between the two different MVPCI strategies concerning MACE and the safety outcomes (length of hospital stay, CIN). Primarily this study suffered from a small sample size.14
A post-hoc analysis of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, originally designed to compare bivalirudin to heparin plus GbIIb/IIIa inhibitors during primary P-PCI, retrospectively evaluated 275 patients undergoing MVPCI during index and 393 patients during staged procedure.15 In this study immediate MVPCI was associated with an increased all-cause mortality (HR 4.1, 95 % CI 1.93–8.86, p<0.0001) and cardiovascular mortality (HR 3.41, 95 % CI 1.35–7.27, p=0.005).15
In order to further address the issue of ideal timing of revascularisation, a recent network meta-analysis was conducted by Elgendy et al. comparing the four treatment strategies.16 The most important quality criteria of included trials were an adequate description of treatment allocation, blinded outcome assessment and description of losses to follow up. In the sensitivity analyses, trials were excluded if they did not include urgent revascularisation as part of MACE, were conducted before 2010, included less than 100 patients, and are yet to be published. A total of 10 trials with 2,285 patients were included in the final analysis. The median FUP time was 25 months (6–38 months). The primary outcome of MACE, defined as in the individual trials, was significantly reduced due to complete revascularisation (index procedure: RR: 0.37, 95 %: CI 0.24–0.59; staged before discharge: RR: 0.49, 95 % CI: 0.27–0.91; staged after discharge: RR 0.58, 95 % CI 0.35–0.97), but there were no differences between the different complete revascularisation strategies. Similar repeat urgent revascularisation was lower in patients fully revascularised, again showing no difference between complete revascularisation at the index procedure or as staged procedure (index procedure: RR: 0.32, 95 % CI: 0.19–0.54; staged before discharge: RR: 0.31, 95 % CI: 0.15–0.65; staged after discharge: RR: 0.46, 95 % CI: 0.25–0.85). There was no difference in the risk of all-cause mortality among the four revascularisation strategies. The authors concluded that each of the three revascularisation strategies has advantages and disadvantages concerning technical and pathophysiological aspects. Furthermore, they stated that 4325 patients (power analysis) would be needed to achieve 80 % power for all-cause mortality.16 Earlier meta-analysis concluded that staged complete revascularisation is associated with the lowest risk of short- and long-term mortality.17,18 However, in contrast to the meta-analysis performed by Elgendy et al., these analyses mainly included observational studies.
In a pre-specified subgroup analysis of the CvLPRIT trial, infarct size and left ventricular function in patients who underwent immediate compared to staged complete revascularisation were assessed.19 In a post hoc analysis cardiac magnetic resonance (CMR) scans were performed before discharge, after any staged procedure and after 9 months in 98 patients.20 Patients who were chosen to have staged MVPCI had more visible IRA thrombus, a higher SYNTAX score and more no-reflow after PCI. These differences in baseline characteristics led to larger infarcts, less myocardial salvage and reduced ejection fraction as detected by CMR. A surprising finding of this study was a higher frequency of N-IRA MI (type 4a MI) detected by CMR in patients treated with staged procedure (40 % versus 14 %, p=0.006), which may be related to a greater number of stents implanted in the staged group and different use of adjunctive medication (bivalirudin, Gp IIb/IIIa inhibitors). However, as these patients were not randomised to immediate or staged procedure and due to differences in baseline characteristics, these results can only be considered hypothesis-generating.20
The aim of the most recent meta-analysis by Pasceri et al. was to assess whether complete revascularisation can reduce hard clinical endpoints such as total mortality and MI and to determine the possible role of timing of revascularisation.21 Eleven randomised trials enrolling 3561 patients were included. Patients who had complete revascularisation (immediate or staged) had a 25 % relative risk reduction of all-cause mortality or MI (p=0.04). Taking all 11 RCTs together, immediate complete revascularisation significantly reduced death or MI when compared to staged procedure (p=0.025).21 A possible explanation made by the authors refers to the pathophysiology of the disease as the risk of adverse events in STEMI is higher within the first few days.3 Thus, achieving complete revascularisation as soon as possible might help to reduce the risk of death or MI. However, none of these trials staged patients within the first two days, leaving the question open if early staging is as good as immediate PCI.
To summarise these findings, there is currently some evidence that complete revascularisation is beneficial compared to a culprit only strategy, although adequately powered RCTs to assess the impact on all-cause mortality are still missing. There is currently no randomised controlled evidence to prefer one MVPCI strategy over the others as head-to-head comparisons in large cohorts are missing.
Furthermore, as most RCTs excluded high risk patients (cardiogenic shock, chronic total occlusion, left main stenosis), evidence in these populations is lacking and they have to be discussed separately.
STEMI Patients Suffering from Cardiogenic Shock and MVCAD
Cardiogenic shock is a serious condition complicating acute MI in 5–10 % of patients and is associated with a short-term mortality of 40–50 %.1 About 80 % of patients suffering from cardiogenic shock have MVCAD, further increasing mortality in these patients.22,23 The main argument in favour of immediate complete PCI in these patients is the improvement of overall myocardial perfusion and function, which is counteracted by the risk of renal impairment due to contrast volume, prolongation of procedure time and volume overload. Meta-analysis of observational trials suggested that immediate MVPCI is harmful in patients with MI and cardiogenic shock and that short-term mortality is higher.23 This result was intriguing as it challenged the 2017 recommendation of the ESC to consider immediate MVPCI in patients with STEMI and cardiogenic shock (expert opinion).
The Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial randomly assigned patients with acute MI (STEMI and NSTEMI) and MVCAD (n=706) to immediate complete or target vessel only PCI (complete revascularisation encouraged). Staged procedure in the latter group was performed on the basis of symptoms of ischaemia, FFR, non-invasive testing, and clinical and neurological status. The study was designed to test the hypothesis that culprit lesion only PPCI is superior in this population. The primary endpoint was a composite of death from any cause and severe renal failure leading to renal replacement therapy within 30 days. The primary endpoint was significantly lower in patients who had target vessel only PCI during index procedure (45.9 versus 55.4 %, RR: 0.83, 95 % CI: 0.71–0.96, p=0.01). Furthermore, relative risk of death (RR: 0.84, 95 % CI: 0.72–0.98, p=0.03) and renal replacement therapy (RR: 0.71, 95 % CI: 0.49–1.03, p=0.07) was reduced in these patients. There were no significant differences in recurrent MI, rehospitalisation for congestive heart failure, bleeding and stroke. The authors concluded that the acute hazards of a prolonged procedure time seem to outweigh any potential negative effects of repeat revascularisation. The main limitations of the study were crossover between groups (although rather infrequent) and the fact that cardiogenic shock management was not standardised.
STEMI Patients Suffering from MVCAD with Chronic Total Occlusion
Chronic total occlusion (TO) is found in 10–15 % of STEMI patients.24 There is currently increasing evidence that worse outcome of patients suffering from MVCAD compared with single vessel disease is mainly driven by CTO. In observational trials, revascularisation of CTO lesions led to improvement of LVF and survival. However, PCI is offered only to a minority of patients with CTO (10 %).25,26
The first RCT powered to show differences in left ventricular ejection fraction (LVEF) and left ventricular end-diastolic volume (LVEDV) was the Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation Myocardial Infarction (EXPLORE) trial.27 The hypothesis behind revascularisation of CTO in STEMI patients was restoration of contractile function and an improved healing of infarct border zone. Patients were randomly assigned to staged PCI of CTO within 7 days after STEMI (n=150) or no PCI of CTO (n=154). There were no differences in the two primary endpoints LVEF (CTO PCI: 44.1 ± 12.2 % versus no CTO PCI: 44.8 ± 11.9 %, p=0.6) and LVEDV (CTO PCI: 215.6 ± 62.5 ml versus no CTO PCI: 212.8 ± 60.3 ml, p=0.7) after 4 months evaluated by cardiac MRI. Furthermore, there was no significant difference in MACE (CTO PCI: 5.4 % versus no CTO PCI: 2.6 %, p=0.25). The adjudicated success rate of CTO PCI was 73 %. However, in a subgroup analysis, PCI in patients with CTO in the left anterior descending artery (LAD) led to a significantly improved LVEF (47.2 ± 12.3 % versus 40.4 ± 11.9 %, p=0.02). An improvement in LVEF was also found in earlier large observational trials.28,29 The main limitations of this trial were the exclusion of high risk patients, a loss of power as the CTO PCI success rate was lower than expected and the trial was not powered to show differences in hard clinical endpoints.
Future Perspectives
The variety of trials conducted leaves us with uncertainty on when and how to perform complete revascularisation in STEMI patients. Furthermore, as all the aforementioned RCTs lack power to show differences in relevant clinical endpoints, there is a need for larger trials to address these issues.
Three ongoing trials will help to further clarify the role of complete revascularisation in STEMI patients with MVCAD. The Complete Versus Culprit-only Revascularization to Treat Multivessel Disease After PCI for STEMI (COMPLETE) trial was designed to randomly assign STEMI patients to staged complete PCI versus optimal medical treatment 72 hours after PPCI. The trial is estimated to be completed at the end of 2018. The primary outcome is a composite of cardiovascular death or new MI, over a FUP period of 4 years (Clinical Trials.gov Identifier: NCT01740479). The MULTIvessel Immediate Versus STAged RevaScularization in Acute Myocardial Infarction (MULTISTARS AMI) trial estimates to enrol 1200 patients comparing complete revascularisation during the index procedure to culprit lesion only PPCI, but subsequent staged revascularisation of all relevant lesions within 19–45 days. The primary outcome of the trial is a composite endpoint of all-cause death, non-fatal myocardial infarction and unplanned ischaemia-driven revascularisation. The estimated study completion date is December 2019 (Clinical Trials.gov Identifier: NCT03135275). Finally, the FULL REVASC (FFR-Guidance for Complete Non-Culprit Revascularization) trial will randomise STEMI patients (n=4052) to FFR-guided non-culprit PCI, either immediately or staged. The study is powered to detect differences in 1-year all-cause mortality and MCI (Clinical Trials.gov Identifier: NCT02862119).
Conclusions
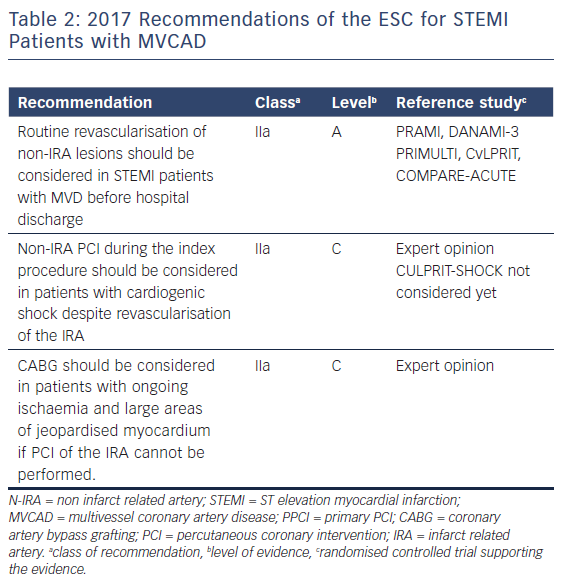
Complete revascularisation should be considered in patients with STEMI and MVCAD. This recommendation, recently introduced in the STEMI Guidelines 2017 of the ESC (Table 2), is mainly driven by a lower rate of repeat revascularisation in these patients. However, the optimal time point of complete revascularisation in haemodynamically stable patients is still a matter of debate. In cardiogenic shock a ‘culprit vessel only’ strategy during PPCI seems to be beneficial, as prolonged procedures in this setting might be hazardous. Although the role of FFR or imaging techniques like cardiac MRI is still not clear, there is little evidence that FFR guided complete revascularisation could be safely done during the index procedure. In complex lesions such as CTO, staged revascularisation did not prove to have any beneficial effects so far, except in a sub-group of patients with CTO of the LAD. As recent RCTs failed to show differences in hard clinical endpoints, further larger trials are needed to answer the question if we should ‘stay or stage’ in patients with STEMI and MVCAD.
Personal Approach
In our own institution, which is part of the Vienna STEMI network, we attempt full revascularisation in a ‘stay AND stage’ fashion, i.e. performing FFR-guided revascularisation of non-culprit lesions in a second sitting within the hospital stay. In stabilised patients, such as those with spontaneously recanalised culprit lesions, there is little reason not to treat an unambiguous tight non-culprit lesion, if proximal and easily accessible. In contrast, patients with complex non-culprit lesions such as left main or complex bifurcations should be treated in a staged procedure. In patients with shock, based on recent data, the decision to treat more than just the culprit lesion should be carefully made based on the morphology and relevant territory of the given lesions.