Detection of obstructive disease of the left main coronary artery (LMCA) is relatively unusual in the catheterisation laboratory, as it accounts for approximately 4 % of all coronary angiograms, with isolated LMCA disease observed in only 5–10 % of these cases.1
Intervention to the LMCA is, however, notable compared to the treatment of coronary stenosis elsewhere in the coronary tree. First, potential complications occurring during LMCA intervention may rapidly progress towards haemodynamic instability, since the LMCA provides the blood supply to 80 % of the left ventricle in patients with right coronary dominance.2 Second, disease of the LMCA is difficult to assess angiographically because of the possible lack of a proximal reference.3 Third, atherosclerosis at the LMCA site is diffuse in many cases, frequently involving bifurcation, and often features a higher rate of fibrotic and calcific components, making LMCA lesions tougher with a consequent need for appropriate and careful lesion preparation.4,5
In the current review we analyse the role of intravascular imaging with intravascular ultrasound (IVUS) as a key step in percutaneous coronary intervention (PCI) to the LMCA in order to achieve an optimal final result.
Percutaneous Coronary Intervention or Coronary Artery Bypass Grafting in LMCA Disease
Historically, coronary artery bypass grafting (CABG) represented the treatment of choice, with a well documented prognostic benefit mainly related to the very high rate of long-term patency of the left internal mammary artery graft.6,7 Data from four large studies have highlighted the potential equivalence of stenting and CABG in the LMCA setting.8–11
In particular, the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) study showed that when coronary anatomy complexity is low or relatively low (defined by a SYNTAX score <32), then CABG and PCI performed well have similar outcomes at 1- and 5-year follow-up, with PCI affected by a higher rate of repeated revascularisation and CABG by an higher rate of stroke.9,12,13 These conclusions led to a change in the guidelines, with PCI having a IB indication for LMCA disease when the SYNTAX score is <22 and IIaB when the SYNTAX score is >22 and <32, with no current indications for PCI in LMCA disease when the SYNTAX score is >32 (IIIB indication).14 These conclusions also prompted initiation and recruitment for the randomised Evaluation of the Xience Everolimus Eluting Stent vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) and Nordic–Baltic–British Left Main Revascularization (NOBLE) studies comparing CABG with PCI for the treatment of LMCA disease.15 These two large recently-completed trials investigated CABG versus ‘state of the art PCI’ (e.g. with fractional flow reserve (FFR) and IVUS guidance and with second-generation drugeluting stent adoption) in patients with LMCA disease and low and intermediate SYNTAX scores. While EXCEL confirmed non-inferiority of PCI in LMCA compared to CABG in terms of major adverse cardiovascular events at 3-year follow-up,16 the NOBLE trial did not show the same level of benefit, with CABG still being superior to PCI.17 The need for longer follow-up in EXCEL (5 years) and several limitations in study design and implementation in NOBLE, whose analysis is beyond the aim of this review, need to be taken into account before considering a change in the guidelines for LMCA disease treatment. In our opinion, there are two clear messages that are consistent across the two studies: the unquestionable role of the heart team in the revascularisation decision-making process and the need for proper lesion preparation and optimisation of the final PCI result.
The potential technical complexity behind LMCA stenting and the proven efficacy of CABG in LMCA disease mean it is essential that when PCI is performed on the LMCA, every effort is made to ensure that the final outcome from revascularisation is at as good as the one potentially achievable with CABG. This requires careful patient selection, rigorous procedural planning and application of the best available technology (in terms of stent and imaging techniques). The use of intravascular imaging, and usually IVUS, is highly recommended during elective LMCA intervention; a recommendation supported by a IIaB indication in the European guidelines.14
IVUS in LMCA PCI
Definition of LMCA Anatomy and Plaque Distribution
Coronary angiography is the initial technique for LMCA assessment, but because of its two-dimensional nature it cannot provide an accurate evaluation of the extent of disease, especially where there is eccentric distribution of the atherosclerotic plaque or complex anatomy with tortuous overlapping segments of the coronary tree. Moreover, a coronary angiogram only provides information about the contrast dyefilled lumen, with no insight into the vessel wall characteristics.18 In ostial LMCA involvement, for example, the coronary angiogram can be difficult to interpret, requiring the operator to rely on indirect signs of LMCA ostium disease such as pressure damping during LMCA intubation or lack of contrast dye spilling back during selective injection.
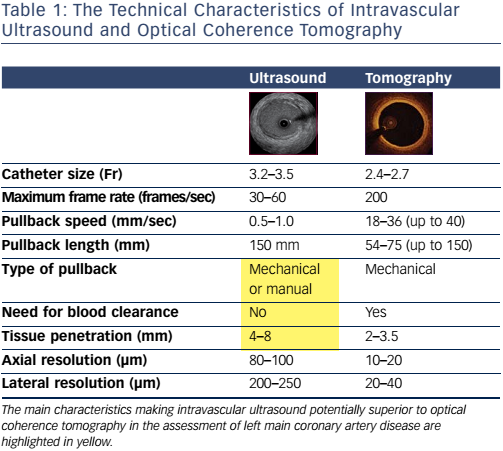
Conversely, IVUS is an accurate technique for assessment of both lumen and wall characteristics.19 The higher tissue penetration of ultrasound compared to infrared light means that IVUS can play a key role in LMCA assessment compared to optical coherence tomography (OCT). IVUS imaging can provide a better visualisation of the LMCA and its ostium than OCT, since it does not require contrast injection to clear the lumen of blood. More detail is available if manual pullback is used, and consequently IVUS can offer superior assessment of the LMCA ostium. For these reasons, although we recognise the value of OCT in distal LMCA assessment,20 we believe that IVUS should probably still be considered the first-line imaging method for LMCA and LMCA ostium assessment. Table 1 summarises the main technical features of these two techniques.
In the pre-fractional flow reserve era, IVUS was used to define the degree of stenosis in angiographically-determined intermediate LMCA disease. Minimal lumen area (MLA) was then adopted as a parameter to determine when intervention to the LMCA could be safely deferred. After the initial cut-off of MLA ≤9.0 mm2, 21 studies started to propose progressively lower thresholds for deferring intervention, until the currently-accepted value of 6 mm2 was validated against FFR by Jasti et al22 and clinically assessed in the Spanish Working Group on Interventional Cardiology (LITRO) study,23 where it showed no difference in 2-year mortality in patients with deferred PCI to the LMCA compared to revascularised ones. Smaller cut-offs (4.8 and 4.5 mm2) have recently been proposed and validated with FFR by other groups, but since they specifically refer to Asian populations, they should not be applied in other ethnic groups.24,25 For this reason, current practice is to defer PCI to the LMCA when MLA >6 mm2.
The reliability of FFR in LMCA disease has been confirmed and it is usually the first-line method for assessing angiographically-determined intermediate LMCA disease. IVUS should be used to plan the way in which PCI should be performed to the LMCA. It is important to highlight that IVUS can still be integrated with FFR in assessing the indication for revascularisation when moderate to significant LMCA disease is present in the left anterior descending (LAD) or left circumflex (LCx) branch of the LMCA bifurcation. Data from studies of animal models and patients have shown the potential to underestimate the true LMCA stenosis using FFR when significant concomitant proximal LAD or proximal LCx disease is present.26,27 In their study, Fearon et al reported a statistically significant, though small and not clinically relevant, discrepancy between FFR values in LMCA disease in the presence and absence of concomitant LAD disease. In conclusion, therefore, FFR is an easy to use and highly effective tool for isolated LMCA disease assessment, while a more critical approach is required in the presence LMCA disease and concomitant disease of the proximal LAD or LCx branch. As the same authors noted, the real challenge is to assess LMCA disease contribution to the overall the ischaemic burden when the FFR measured in the less diseased branch is in a ‘grey zone’ between 0.85 and 0.81.27 In this context, implementation with IVUS could help the operator decide on the indication for LMCA revascularisation.
When angiography is ambiguous, IVUS pullback should be performed on both branches of the LMCA bifurcation, or at least on the branch with the lower degree of angiographic disease. This approach will produce information about the distribution of atherosclerosis at the site of the bifurcation, as well as at the ostium of the LMCA. LMCA disease frequently involves both branches of the bifurcation, even when angiography appears to be ‘normal’.28 Understanding the exact distribution of plaque burden at the bifurcation is clearly important when deciding whether a provisional stenting strategy can be applied to treat LMCA disease or a two-stent strategy should be considered upfront. In this regard, a MLA <3.7 mm2 or plaque burden >56 % in the LCx ostium has been shown to predict the need for a second stent after provisional stenting of the main vessel.29
It is important to highlight that although no large randomised clinical trial has been performed to assess whether intravascular imaging-guided PCI to the LMCA is associated with better long-term clinical outcomes, there are convincing data from large registries to suggest long-term mortality benefit in patients undergoing IVUS-guided compared to angio-guided PCI to the LMCA.30,31 Careful analysis of IVUS data from both the EXCEL and NOBLE trials will be pivotal in confirming the value of intravascular imaging in assisting LMCA PCI. Currently, it is only possible speculate that systematic IVUS could corroborate and improve on the promising results of PCI on the LMCA at 10-year follow-up described by Sheiban et al in an international registry with a IVUS adoption of only 14 %.32 This particular aspect is relevant when considering the cost-effectiveness of IVUS imaging. Notably, the costeffectiveness analysis derived from SYNTAX I confirmed the clinical and economic benefits of LMCA PCI over CABG specifically in the subgroup of patients with low (<22) SYNTAX scores.33
Qualitative Characterisation of LMCA Disease
Its ability to image the deeper layers of the arterial wall make IVUS the best technique for the qualitative assessment of plaque composition at the site of the LMCA. As a general rule, lesion preparation is pivotal in LMCA PCI. Calcium is expected and a low threshold for considering rotational atherectomy34 is encouraged to prepare the LMCA lesion. In this context IVUS defines the extension of calcium35 by the high backscattering signal with posterior shadowing.36
Visualisation of extension of the calcific arch is attainable with IVUS; a calcific arch >180° is a strong indication for rotational atherectomy. Pre-stenting IVUS can help differentiate cases in whom rotational atherectomy is mandatory from those in whom predilation with noncompliant balloons at high pressure could be a possible alternative, especially in those catheter laboratories with no operators trained in rotational atherectomy. Besides identifying its circular and longitudinal extension, IVUS can also help identify the depth of the calcific component.37 This is relevant, as deep and thick calcium might be associated with a higher risk of coronary perforation during balloon inflation and thus mandate rotational atherectomy.38
Stent Sizing
Having clarified plaque burden, distribution and composition, IVUS can provide information about true vessel dimensions in order to facilitate stent sizing. Due to its large calibre and the mismatch in diameter between the LMCA and LMCA-bifurcation branches, stent sizing in LMCA PCI can be tricky using angiography alone; the operator is called on to make a decision according to the information provided by a two-dimensional technique. Conversely, IVUS-defined lumen area can provide a more detailed definition of the lumen, with stent-sizing that takes into account not just one single diameter (as in angiography), but three diameters, namely the maximum, minimum and mean diameter.
By more accurately detecting atherosclerosis, IVUS can assure a better definition of the proximal and distal references for stent diameter-sizing and better identification of proximal and distal landing zones that are free from disease, thus minimising the risk of longitudinal miss during stent deployment. Consequently, we believe IVUS should be applied before stenting in order to aid proper planning of PCI to the LMCA as it can define LMCA anatomy and plaque burden distribution, characterise disease, especially the extent of calcification, and aid accurate stent sizing. The use of IVUS post-stenting is mainly to check that the stent application is optimal and plaque is covered.
Stent Optimisation
Stent optimisation is the main indication for IVUS in LMCA PCI, and in our opinion post-stenting IVUS should always be performed when it is safe to do so. Although there are no large randomised clinical trials to assess ad hoc whether intravascular imaging-guided PCI to the LMCA is associated with a better long-term clinical outcome, convincing data from large registries suggest a long-term mortality benefit in patients undergoing IVUS-guided compared to angio-guided PCI to the LMCA.30,31
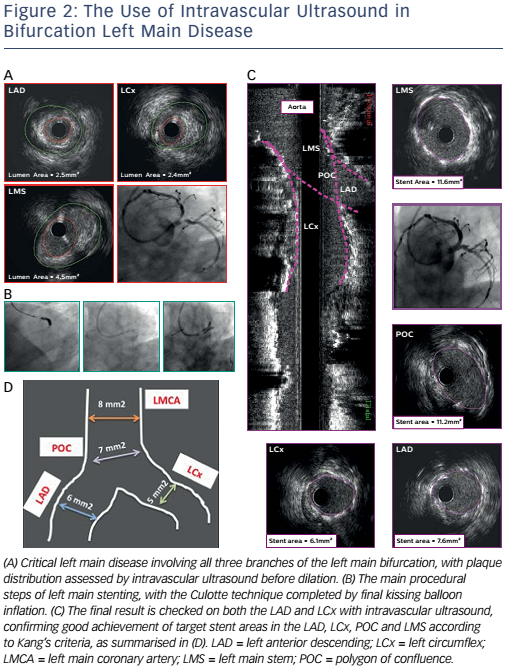
Stent malapposition, stent under-expansion, geographical miss and large uncovered stent-edge dissection are all possible stent-related complications detectable by IVUS.39 Stent under-expansion is the main predictor of stent failure and, as recently reported by Nerlekar et al, IVUS-assisted LMCA PCI can be associated with a lower rate of target lesion revascularisation and stent thrombosis.40 For this reason Kang et al have proposed a minimum area that should be covered in each segment of the LMCA bifurcation after stenting (>5 mm2 at the ostium of the LCx; >6 mm2 at the ostium of the LAD; >7 mm2 at the polygon of confluence; and >8 mm2 at the LMCA).41 The decision to intervene in a case of stent malapposition or edge dissection is more complex as no proven thresholds have been proposed and prospectively validated.
When treating LMCA bifurcation, IVUS can be used to identify the mechanism of side-branch compromise after main-branch stenting.
It is important to recognise whether intervention on the side branch can be avoided if safe and efficacious; IVUS has a role in defining how the operator should eventually intervene. If carina shift is identified as the underlying mechanism, then kissing balloon inflation could be enough to reshape the carina and restore normal flow down both limbs of the bifurcation. Conversely, if plaque shift is detected then the operator may switch from the provisional single-stent strategy to a two-stent procedure.
Case Studies
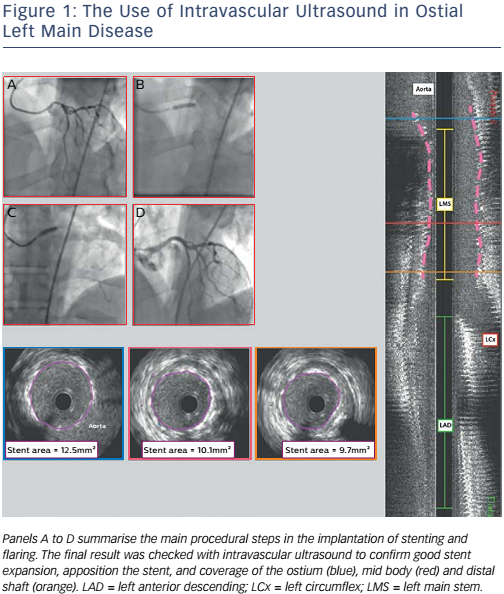
Figures 1 and 2 illustrate the employment of IVUS guidance. Figure 1 shows a case of ostial left main disease in which IVUS was used to confirm stent expansion, stent apposition and coverage of the ostium, mid body and distal shaft. Figure 2 shows left main disease involving all three branches of the left main bifurcation. The use of IVUS enabled the assessment of plaque distribution and confirmed the achievement of Kang’s criteria for minimum stent areas in the LAD, LCx, polygon of confluence and left main stem.41
The Workflow Algorithm
Since IVUS is configured to be a mandatory step in an already complex and potentially risky procedure such as PCI to the LMCA, it is desirable that its application is easy and user-friendly. For this reason it is extremely helpful having a workflow algorithm to guide the operator at each step of the intervention, particularly in the collection of IVUSderived data considered necessary for the decision-making process and procedure planning. Here, we propose a workflow algorithm to facilitate IVUS applications during LMCA.
After having defined LMCA disease according to angiographic appearance, this algorithm encourages the operator to perform an IVUS pullback. Ideally IVUS pullback should be performed in both branches of the LMCA bifurcation, however where this is not possible it should be performed in the least diseased one. If angiographic intermediate disease is observed, FFR is advised in addition to IVUS pullback. The algorithm helps the operator collect only the data strictly necessary for planning the procedure, making IVUS interpretation quicker and more straightforward.
Nine possible scenarios are identified according to the presence of MLA <6.0 mm2 in the LMCA, or MLA <4.0 mm2 or plaque burden >70 % in one or both bifurcation limbs. These nine scenarios are:
- no LMCA disease;
- LMCA disease with no bifurcation involvement;
- LMCA disease with Medina classification 1,0,0 bifurcation involvement;
- LMCA disease with Medina 1,1,0 bifurcation involvement;
- LMCA disease with Medina 0,1,0; bifurcation involvement;
- LMCA disease with Medina 1,0,1 bifurcation involvement;
- LMCA disease with Medina 0,0,1 bifurcation involvement;
- LMCA disease with Medina 0,1,1 bifurcation involvement;
- LMCA disease with Medina 1,1,1 bifurcation involvement.
The presence of right or left dominance is another parameter the algorithm takes into consideration. For each specific scenario, the algorithm provides an action plan suggesting the appropriate stent technique and FFR and IVUS guidance to optimise the result of stenting. For further details about the workflow, we refer the reader to the following link http://bit.ly/2qG69nx.
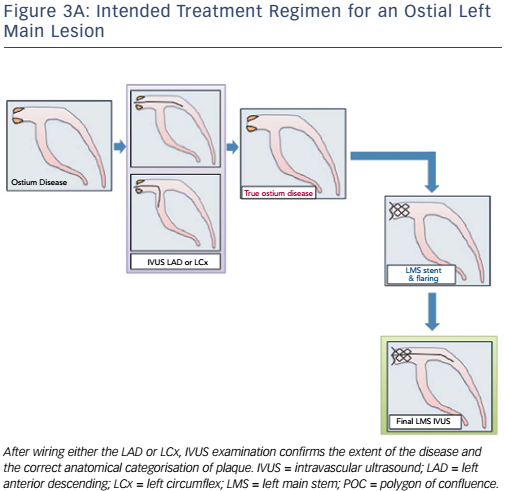
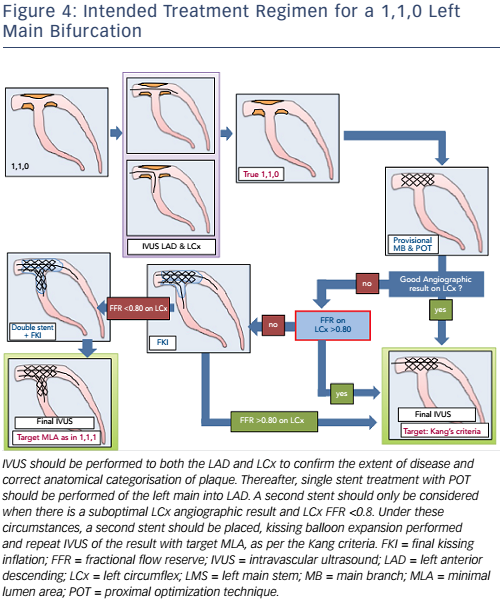
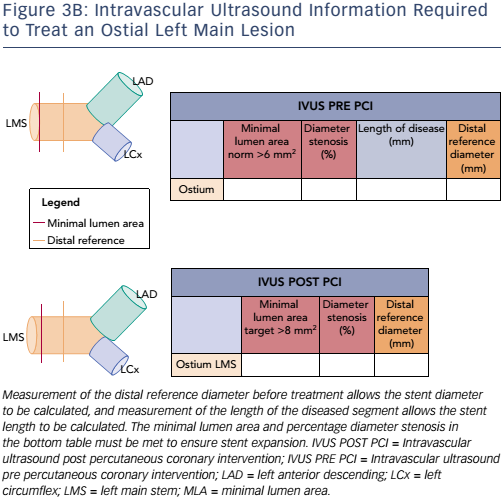
Currently, the clinical application and validation of this workflow algorithm is under investigation, but it has been designed as a tool to encourage IVUS adoption during LMCA PCI in agreement with European guidelines. Representative examples of the IVUS algorithm are shown in Figures 3A and B (ostial left main disease) and Figure 4 (Medina 1,1,0 left main bifurcation).
Conclusion
In conclusion, IVUS has become a standard part of the PCI procedure for the treatment of LMCA disease. Improving the consistency of its application is likely to improve stenting technique and results, and consequently patient outcomes.