Coronary artery bypass grafting (CABG) surgery is a common method of coronary revascularisation and remains the standard of care in patients with multivessel coronary artery disease (CAD), as well as in those with diabetes.1 Since 2004, CABG numbers have been in decline in the UK, whereas the number of percutaneous coronary interventions (PCI) has shown a consistent increase year-on-year until a recent plateau.2 Post-CABG patients now represent a significant proportion of patients who subsequently require PCI and represent a challenging cohort in terms of clinical frailty and anatomical complexity.3 In this review we discuss these challenges and specifically consider the subset of post-CABG patients presenting with chronic total occlusions (CTO) in their native coronary arteries.
Challenges in Post-CABG Patients
Graft Failure
Although surgical success rates remain high, venous bypass graft patency rates remain the ‘Achilles’ heel’ of the long-term prognosis of surgical revascularisation. Whereas arterial revascularisation has been demonstrated as a superior, lasting method,4–6 venous bypass grafts do not withstand this test of time. Left internal mammary artery (LIMA) grafts can remain patent in 88–100% of patients at 15 years,7,8 a finding echoed by right internal mammary artery (RIMA) use, which retains excellent graft patency up to 10 years, with patency rates quoted at 81% or equivalent to the LIMA for identical coronary territories.8,9 However, saphenous vein grafts (SVG) have relatively poor patency rates.10,11 In their meta-analysis, Athanasiou et al. compared SVG patency with radial artery graft patency.12 Of the seven studies examining patency rates after a median 5-year follow-up, four recorded SVG patency rates between 65% and 72%, whereas the others recorded higher rates (72–91%).12 In a study of 1,074 patients, 10-year SVG patency rates were 61% when compared to LIMA grafts, where the patency rate was recorded at 85%.13
More contemporary data are available from the COMPASS-CABG substudy and POPular CABG trials, both of which used CT coronary angiography (CTCA) to assess SVG patency 1 year after surgery.14,15 In the COMPASS-CABG substudy, patients were treated with rivaroxaban, with or without aspirin or aspirin alone, with an overall 9.6% occlusion rate of all SVG studied.14 In the POPular CABG trials, graft occlusion occurred in 9.9% of all grafts, with no significant improvement despite the addition of ticagrelor antiplatelet therapy.15 CTCA has allowed non-invasive assessment of graft patency, and its wider use may uncover further aspects of post-surgical coronary anatomy and graft viability not previously appreciated.16,17
In particular, attention should be paid to the increased likelihood that patients with existing CTOs and multivessel disease possess higher anatomical and clinical risk scores and are thus more likely to be referred for CABG in the first instance.18–20 Yet, postoperative angiographic assessment in patients who underwent both on- and off-pump CABG for CTOs has revealed that grafts placed on non-left anterior descending artery (LAD) collateralised CTOs suffer from extremely poor patency rates at 1-year follow-up, as low as 22–24%, which is an unacceptably low graft viability rate that should call into question the rationale for CABG in the presence of a non-LAD CTO.21
Revascularisation Complexity
Christopoulos et al. describe the post-CABG population as older, more likely to suffer from diabetes and have suffered from previous MI.22 CTOs are more prevalent in this subgroup of patients than in those without prior CABG, with registry data demonstrating the presence of a CTO in 54% of evaluated post-CABG patients.23 Patients with a CTO and symptoms relating to ischaemia with myocardial viability do benefit from revascularisation versus optimal medical therapy alone, with improvements in both symptom burden and quality of life.24 However, they are less likely to receive revascularisation therapy, likely due, in part, to the perceived complexity of the procedure.23 In the Canadian Multicenter CTO Registry published by Fefer et al., of 1,697 patients identified with a CTO (and no prior CABG), medical therapy was opted for in 44% of patients, with 26% undergoing CABG (89% had a bypass graft on the CTO vessel) and 30% undergoing PCI. CTO PCI was attempted in only 31% of these patients and CTO success was achieved in only 24% of all patients undergoing PCI.23 This registry (2008–2009) suggested the presence of CTOs to be approximately 18% of all patients with CAD, and yet just under half these patients received medical therapy alone, one-quarter received surgical revascularisation and the remainder underwent PCI.23 This ‘interventional paradox’ will see some patients denied revascularisation for symptoms due to anatomical complexity and the perceived complexity of PCI. Furthermore, post-CABG patients will represent additional challenges when re-presenting with angina pectoris: they are likely to be older, have more comorbidities and have more complex coronary lesion characteristics, and for many, repeat CABG is not feasible due to excessive surgical risk compared with CABG-naïve patients.3,25–30
Saphenous Vein Graft Intervention
Therefore, in post-CABG patients, PCI remains the only strategy for repeat revascularisation, yet the presence of a previous bypass graft creates additional challenges to conventional PCI. While medical therapy can be a good first option for the treatment of angina, PCI for moderate SVG stenoses when compared to optimal medical therapy (OMT) can be effective, with lower rates of major adverse cardiovascular events (MACE) at 1-year follow-up in the VELETI I trial.31–33 Although the VELETI I trial was a hypothesis-generating, small, randomised pilot trial, it put forward the concept of ‘plaque sealing’ of moderate, non-significant atheromata in SVGs, which are thought to undergo accelerated atherosclerotic disease progression compared with native vessels.33 The subsequent larger randomised controlled VELETI II trial did not demonstrate any reduction in clinical endpoints in SVG PCI with drug-eluting stents (DES) at the 3-year follow-up compared with OMT in these so-called ‘intermediate’ lesions, although the pooled analysis of both VELETI trials may yet support the controversial concept of plaque sealing.34,35
Percutaneous treatment of SVGs accounts for between 5% and 10% of all PCIs.36–42 Although, unsurprisingly, the vast majority of SVG PCIs are performed within the body of the graft, approximately one-fifth of graft lesions occur at the aorto-ostial anastomosis and one-sixth occur at the distal anastomosis.43 Acute thrombotic SVG occlusion must be managed in the same manner as native coronary occlusion and, although procedural success tends to be high, mortality, recurrent acute coronary syndrome (ACS) and the need for revascularisation within the short to medium term remains significant.42,44 Preference is given to revascularising the native coronary artery over SVG by existing guidelines on myocardial revascularisation.1 The paucity of data for this recommendation has led to development of the PROCTOR trial, a multicentre, multinational European randomised control trial, which will randomise patients to native vessel or SVG PCI, with results expected in 2027.45
The physiology of SVG failure is not fully understood, but it is thought these grafts are poorly adapted to arterial flow and the pathobiology of SVG degeneration results in a friable vessel with atheromatous debris to contend with.46,47 Additional challenges include the potential for embolisation of this debris into distal epicardial and coronary microcirculatory vasculature, resulting in the plugging of capillaries, increasing the prospect of the no-reflow phenomenon and associated risk of MI and subsequent in-hospital mortality.48–50 The routine use of distal embolic protection devices (DPD) has shown potential to significantly reduce periprocedural MI rates, but no significant reduction in in-hospital mortality could be demonstrated.51–54 However, these devices are cumbersome to deploy and, as such, their use has been historically limited.55,56 Furthermore, several observational studies and large registry data have shown conflicting results.57,58 Thus, the strength of recommendation for the use of DPD for SVG PCI was downgraded in the most recent update of the European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularisation to a Class IIa, level of evidence B, recommendation.1 Female sex, lesion length, extensive degenerative change and high plaque volume in diseased SVGs predict 30-day MACE.59,60
Periprocedural MI (as defined by a rise in creatine kinase (CK)-MB between ×1 and ×5 the upper limit of normal) was a stronger predictor of adverse outcome than similar cardiac enzyme values following SVG PCI and a powerful predictor of late cardiovascular mortality in an albeit dated study, with the overall rate of periprocedural MI reported as 15%.61 Periprocedural increases in CK-MB following SVG PCI are unsurprisingly significantly greater when no-reflow occurs (43% versus 4%, p<0.001) with probable thrombus, ACS presentation, graft degeneration and graft ulceration independent predictors of no-reflow.48 More contemporary studies of SVG PCI tend to use DPD, such as the post hoc analysis of the DIVA trial comparing direct stenting against stent deployment with or without balloon inflation (either prior to and/or after stent implantation).62 Patients were recruited to the DIVA trial between 2012 and 2015 and DPD use was >70% in both groups. Rates of periprocedural MI were low, at 4% of total lesions treated and 5% of patients treated.62 The use of DES in SVGs is now supported by a number of trials, all demonstrating poor longevity following treatment with plain old balloon angioplasty and covered stents.63–66 DES is advocated for SVG PCI due to lower rates of repeat revascularisation compared with the use of bare metal stents, although clinical outcome data remain conflicted, with only a limited number of randomised trials available.1,52,53,67–69 In the absence of randomised control data comparing SVG and native vessel PCI, registry data suggest SVG PCI remains inferior to native vessel PCI, with higher MACE rates, principally driven by MI and revascularisation rates, at 1 year.70 A history of previous bypass graft surgery is associated with a higher risk of restenosis, and SVG as the PCI target is independently associated with an increased risk of very-late stent thrombosis.71,72
Chronic Total Occlusion Revascularisation in Post-CABG Patients
Although there remains a paucity of data from randomised control trials supporting CTO revascularisation, symptom- and, where relevant, myocardial viability-driven revascularisation has been established by the EuroCTO Trial.24 This approach is supported by the latest guidelines.1 The DECISION-CTO trial did not report an improvement in quality of life outcomes, although this trial fell short in recruitment and thus was stopped early.73 Although the trialists have been congratulated for the large number of patients randomised, several limitations have been identified, including a lack of baseline symptoms, cross-over to the CTO PCI group (from the non-CTO PCI group) and the non-inferiority and pre-PCI randomisation design, in addition to the underpowered study.74
CTOs in the presence of bypass grafts are often longer in length with a higher calcific burden and diffuse atherosclerotic disease.75,76 These CTOs are themselves complex, as graded by the frequently adopted Multicenter CTO Registry of Japan (J-CTO) score, with higher J-CTO scores than in non-CABG patients, and suffer from greater anatomic distortion, with three-dimensional tenting effects exerted on the native vessel at the distal graft anastomosis.22,28,77–81 It is unclear whether this is the result of a pre-existing heavy burden of disease that necessitated CABG revascularisation in the first instance, accelerated atherosclerosis or the presence of the distal graft anastomosis resulting in disease progression due to competitive flow.82–86 In addition to these anatomical and pathophysiological factors, patient characteristics must also be considered. Evolution of knowledge, techniques and, perhaps most importantly, equipment has facilitated higher rates of success in CTO revascularisation. Among these, the introduction of microcatheters has dramatically altered the ability of operators to safely and successfully cross CTOs and these should be used in all CTO PCI cases regardless of complexity. Microcatheters are further elaborated on below.
Revascularisation by redo CABG in patients with prior CABG is not without jeopardy, with a two- to fourfold increased risk compared with first-time CABG.87,88 However, mortality was comparable between PCI and redo-CABG for these patients at 3 years, with higher rates of revascularisation in PCI patients.39,89,90 Post-CABG patients can suffer cardiac tamponade at the same frequency as non-CABG patients.91 In addition, prior CABG is associated with reduced event-free survival, with higher rates of cardiac death and MACE demonstrated by univariable analysis and higher rates of MACE demonstrated by multivariable analysis, driven largely by target vessel revascularisation.28 ‘Dry tamponade’ has been recognised as a significant complication of coronary perforation in post-CABG patients, caused by the extravasation of blood within the myocardial wall or adjacent structures within a pericardium with more adhesions.92,93 In-hospital complications are also more frequent in prior CABG patients undergoing CTO PCI than in non-CABG patients, as reported in a multicentre registry of 2,058 patients (prior CABG n=401; non-CABG n=1,657), with higher rates of major complications (3.7% versus 1.5%), any perforation (12% versus 5.2%), periprocedural MI (2.0% versus 0.5%) and procedure-related deaths (0.8% versus 0.1%).27
In a smaller study of 470 patients, contrast-induced nephropathy was more common in prior CABG patients (4.6% versus 1.0%).28 Risk scores developed to predict CTO PCI success, such as the RECHARGE-Score and Clinical and Lesion-related score (CL Score), attribute higher scores to post-CABG patients, reflecting these adverse events.94,95 Of note, previous CABG will preclude rapid and safe sternotomy if a complication arises following or during PCI, and this may have contributed to some of the morbidity seen in these scoring systems.96 Having a sufficiently experienced team to manage complications in post-cardiotomy patients in high-volume PCI centres is essential. The recognition of longer procedures and older and potentially frailer patients with reduced renal function should be considered when evaluating the benefits of potential percutaneous CTO revascularisation, and the ways in which this can be mitigated are further elaborated on below. Conversely, the presence of patent grafts, in addition to providing potential retrograde conduits, can reduce ischaemia in the distal target territory and, in the case of a patent LIMA, reduce the consequences of anterior wall ischaemia from inadvertent left main coronary artery dissection.
Factoring in prior CABG, the presence of a non-proximal lesion position, proximal tortuosity (moderate/severe) and distal cap ambiguity, described as the ‘J-CTO+ model’ improved the power of the J-CTO score in predicting successful CTO crossing.78 These factors provide additional challenges over and above what may be encountered in native vessel CTO PCI in the absence of graft anastomoses. Understanding these potential challenges up front allows the operator to select appropriate techniques and tools to approach CTO cases where SVGs are involved.
Since early pioneers such as Kaltenbach and Reifart in Frankfurt and Rutherford in Kansas City described their experiences in CTO treatment, significant advances in the understanding of pathology, technology and the formulation of accepted standards and techniques have been made, resulting in significantly improved long-term treatment success rates.94,97,98 Original descriptions of CTO PCI were fraught, with difficult, long procedures and prohibitively high reocclusion rates.99,100 Early concepts led to the subsequent development of contemporary tools now in use. The formation of ‘CTO Clubs’, such as the Japanese CTO Club in 1991 and the European equivalent in 2006, improved the sharing and dissemination of knowledge and the development of techniques to improve success rates and reduce periprocedural morbidity. The development of registries such as PROGRESS-CTO and RECHARGE, randomised trials and regional consensus documents have provided a basis for understanding accepted techniques and monitoring contemporary practice, including complication and morbidity rates.24,73,94,101,102 Among these developments, the hybrid algorithm is the currently accepted consensus strategy being used by high-volume, experienced leaders in the field.103 This demands the ability to adopt both antegrade and retrograde approaches to CTO crossing to ensure the optimal use of available techniques with contemporary equipment, with further results from adopting this approach still being reported.
The RECHARGE Registry is thus far the largest of its kind, with over 1,200 patients recruited from European centres to demonstrate both high procedural success rates and low adverse event rates when the hybrid algorithm has been used by experienced operators in high-volume centres.97 It is therefore important to recognise the potential benefits gained through the ability to adopt different strategies to recanalise a CTO and, most pertinently, recognise when to opt for a specific strategy. Retrograde techniques are most often necessary in post-CABG patients due to the complexities described above, usually in conjunction with antegrade techniques that then form the hybrid algorithm approach to these patients.22 Retrograde crossing is more common in post-CABG patients where the SVG can often be used as the collateral channel.22
Whether the native vessel CTO or, indeed, the SVG should be treated in post-CABG patients with graft degeneration remains unclear. Current ESC guidelines recommend PCI as the preferred method of revascularisation in patients with a large burden of ischaemia or severe symptoms due to disease progression or late graft failure.1 However, evidence to support this position is sparse, with limited data implicating prior CABG with poor outcomes, as discussed above, with patient clinical characteristics rather than revascularisation method predominantly determining outcome and anatomical considerations dictating the method of revascularisation.29,71,72 Retrospective and comparative studies have attempted to address this.70,104 However, the PROCTOR trial will be the first randomised trial comparing SVG PCI to native vessel PCI and should help in the decision making for patients with SVG degeneration and stenosis.45
Antegrade Techniques
Operators should be able to call on existing, established techniques of antegrade CTO crossing. These will include antegrade wire escalation (AWE) and/or antegrade dissection re-entry (ADR). Some of these techniques are highlighted below, with supporting evidence discussed.
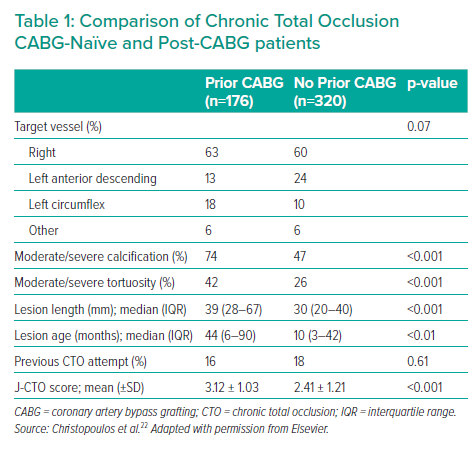
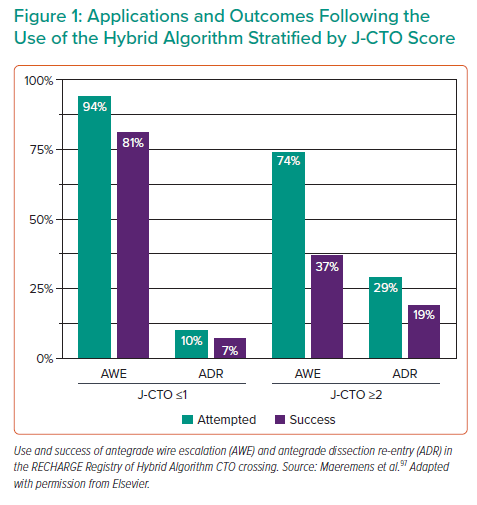
CTOs in the post-CABG cohort exhibit a higher calcific burden, increased tortuosity, longer lesion length and established occlusions for a longer duration, resulting in higher J-CTO scores22 (Table 1).22,76 Although AWE can be a successful strategy and is the default in most cases of CTO, particularly when J-CTO scores are ≤1 (Figure 1), the increased complexity likely to be present in post-CABG patients will often necessitate additional and adjunctive hybrid algorithm techniques.78,97 Antegrade techniques are highly useful in many CTO cases with differences in pathology within the CTO body, but must contend with the presence of more complex, calcified distortions of the artery with severe negative remodelling present than in short-duration CTOs in patients without prior CABG.76 In the RECHARGE Registry, less complex lesions (J-CTO score ≤1) were successfully crossed using an AWE approach with high success rates (86%), whereas ADR and retrograde techniques were often used as bailout strategies with reasonable success.97 However, in more complex lesions (J-CTO score ≥2), AWE was a less successful strategy (50%), requiring ADR and retrograde bailout approaches more frequently.97
Planning Revascularisation
Up-front careful analysis of the coronary angiogram is key to understand potential challenges likely to be encountered during the CTO PCI and can improve success rates considerably.105 The coronary angiogram for the CTO should be acquired without digital magnification in order to visualise the entire course of the vessel, with a long acquisition allowing full visualisation of any antegrade flow either through the CTO or antegrade bridging collaterals into the distal vessel. Large side branches and relevant bifurcations should be noted to help decide which strategy should be used. Where graft anastomoses are present beyond the distal cap of the CTO and where the graft remains patent, antegrade injection along the graft (again without digital magnification) should be used to better visualise the course of the vessel, although this may not be fully appreciated by invasive coronary angiography alone. If dual catheter injections are possible, simultaneous injections first down the patent graft, followed by the native coronary, can provide useful information on occlusion length and potential distal landing zones should an ADR (or, indeed, retrograde) strategy be used. Complex revascularisation attempts, particularly when prior failure has occurred, should be discussed with experienced CTO operators in high-volume centres where familiarity with the hybrid algorithm can be used to establish higher success rates.78,97
Growing evidence supports the use of CTCA as an effective tool for CTO procedural planning in both CABG-naïve and post-CABG patients.106–108 However, CT can have limitations here, particularly when high calcium burdens are encountered, making interpretation challenging, which is more likely in post-CABG patients.43,109 The CT-RECTOR study assessed the predictive value of successful CTO crossing with prior CTCA and was validated against the J-CTO score, suggesting the CT-RECTOR scoring system provided additive data aiding a successful procedure and optimising procedural time.107,108 However, post-CABG patients comprised only 17% and 11% of those included in the studies and, as such, the data should be interpreted with caution in this cohort.
CTOs that have developed in post-CABG patients may have developed multiple native collaterals prior to or since graft degeneration and, as such, visualising the distal vessel may prove challenging. This can be overcome by using retrograde injections from both the diseased graft and contralateral native coronary artery, necessitating the use of an additional vascular access point and a third guide catheter.
Wires
Antegrade techniques use advances in coronary angioplasty wire technology allowing greater options for the operator. These improvements provide the operator with wires with greater torque, steerability and tactile feedback, as well as improvements in wire tip force, and thus greater potential penetration strength. Wire escalation and success in this manner depends on a good understanding of wire properties. Wires will, in general, be hydrophobic, hydrophilic or polymer jacketed, with the latter providing the greatest lubricity, with the pay-off a reduction in tactile feel. Some wires will combine these features, allowing a balance of both ‘slip’ through lesions while allowing the tip to grip lesions and still provide some tactile feedback. It is usual to advance a ‘workhorse’ wire to the lesion, then escalate by exchanging to an appropriate wire, determined by the operator’s appreciation of the occlusion characteristics.
Microchannels and loose tissue through the body of the CTO may be accessed via the proximal cap and, as such, a light yet slippery (hydrophilic or polymer-jacketed) wire with high torque response may be selected to successfully traverse the CTO. Histological findings from a sudden coronary death registry have provided insights into CTO lesion morphology in individuals with and without prior CABG.76 Histological parameters were used to further subdivide CTOs into those with histological parameters suggestive of a ‘short’ or ‘long’ duration and compared with those present in individuals where CABG had been performed at least 2 years prior to autopsy. Although no significant difference was demonstrated between these individuals, a tapered distal cap was more commonly reported in CTOs in prior CABG individuals, whereas an abrupt pattern was noted in the proximal cap, a finding also noted in the ‘long’-duration CTOs examined.76 Tapered proximal occlusions feature loose fibrous tissue with small microvessel recanalisation and so may be more amenable to wire crossing.110 Therefore, a retrograde approach to cross the cap and access the CTO body may be required in prior CABG patients, with heavier, more penetrative wires necessary to cross abrupt (blunt) caps. Gaia wires (Asahi Intecc) are a dedicated family of CTO wires that improve penetration while retaining tactile feedback due to their design featuring a ‘microcone’ tip.111
Heavier tip force and penetrative wires may be required to engage and cross the proximal and distal caps, which are formed of denser tissue than the body of the CTO. Tip loads vary from workhorse wires, which are typically ≤1 g, to gradual increases in tip loads as high as 40 g with the Astato XS 40 wire (Asahi Intecc), which delivers an equivalent penetration force of 796.2 kg/inches2.79 Caution must be exercised when traversing the CTO body with highly penetrative wires, particularly in tortuous and ambiguous vessels where tactile feedback is at a minimum. This is more apparent in post-CABG patients, where the distal graft anastomosis can alter vessel anatomy and result in tenting of the distal vessel. AWE demands an appreciation for wire properties so they are selected to tackle anticipated challenges likely to be encountered. Furthermore, AWE demands an understanding of when to escalate, when to de-escalate and subsequently when re-escalate, if and when appropriate. More detailed information regarding the specifics of wire choice when escalating in an antegrade fashion is available in the antegrade CTO book by Spratt et al.79
Antegrade Dissection Re-entry
When the CTO plaque cannot be traversed through the proximal cap or through the body of the CTO due to obstructions through an antegrade manner, it is often necessary to switch to an ADR strategy. The higher burden of calcium in post-CABG patients may represent one of these obstructions that cannot, despite the use of high-tip-force wires and adjuncts (elaborated on below), be crossed through in a true lumen-to-lumen fashion.76 To perform ADR, the subintimal space must be accessed, and an ‘umbrella’ shape is often used on a polymer-jacketed wire to drive the wire forward in a knuckle fashion to access the subintimal space with relative safety. Caution should be exercised when the subintimal space is accessed towards the distal graft anastomosis to ensure the dissection plane does not extend to or beyond this anastomosis, creating haematoma and thereby potentially occluding graft flow into the distal vessel. The plane of dissection created in this manner should be kept to a short distance from this area and re-entry into the distal true lumen should be attempted in a previously identified distal landing zone. This can be facilitated by using the Bridgepoint System (CrossBoss coronary catheter and Stingray LP CTO re-entry system; Boston Scientific), which provides a more controlled manner with which to advance equipment through the subintimal space with a smaller dissection plane created and targeted re-entry into the distal lumen, demonstrating higher rates of success than the less controlled knuckle wire technique.97
Dual-injection angiography allows an appropriate distal landing zone to be chosen, ideally proximal to the distal graft anastomosis so as not to compromise graft flow (when patent). ADR may not be the ideal strategy when the re-entry zone from the subintimal space back into the true vessel lumen is within 10 mm before the distal graft anastomosis or important side branches due to the risk of extension of the dissection plane and the resulting occlusion of these branches.112 Whether the subintimal space is accessed intentionally during ADR or inadvertently during attempted AWE, antegrade contrast injections should be avoided in order to minimise hydraulic extension of the subintimal space, resulting in compression of the true lumen and thereby reducing the likelihood of successful re-entry.80 Techniques, such as STAR (Sub-intimal TrAcking and Reentry) and LAST (Limited Antegrade Subintimal Tracking), are recognised alternative techniques to traverse the subintimal space and then re-enter into the distal lumen, but are not favoured over the CrossBoss/Stingray system due to a lack of predictable longer-term success.113–115 It is important to recognise the need for at least a 7 Fr system to facilitate the passage and exchange of ADR equipment.
Caution must be exercised when using ADR near side branches. Wires, and subsequently microcatheters, will tend to follow the path of least resistance and, as such, can follow subintimal tracks to enter and dissect side branches, particularly hazardous when antegrade contrast injections are prohibited and so these branches cannot be adequately visualised. Targeted re-entry by identifying a suitable distal landing zone for luminal re-entry and utilising the Stingray balloon, for instance, can help avoid dissection extension and side branch compromise. The CrossBoss catheter features a blunt, atraumatic, 1.0 mm tip and can safely traverse the subintimal plane, but it should not be used as an initial strategy to engage the proximal cap, particularly when ambiguous with multiple bridging collaterals, in the presence of extreme vessel tortuosity or when the vessel course is unclear. The use of a knuckled wire, guide catheter support systems (discussed below) and anchor balloons in proximal side branches can enhance support to enable passage of the CrossBoss catheter and delivery of the Stingray balloon. Once in the subintimal space, the CrossBoss will rarely exit due to the low resistance of the surrounding structures, but short segments of intimal tracking can be evident.80,97
The CrossBoss catheter is not steerable and, as such, advancement through the target vessel structure should be regularly monitored with non-contrast fluoroscopy in orthogonal planes during controlled advancement. Retrograde injections can be of use to ensure the CrossBoss moves in synchrony with the architecture of the visualised distal vessel, so-called ‘dancing’ with the target zone for luminal re-entry. The CrossBoss will track the outer curve of the vessel and, as such, can pass into small side branches, which, if unrecognised prior to further advancement, can result in vessel exit and coronary perforation, a non-negligible complication contributing to a high burden of morbidity and mortality in this small minority of patients.116 In the event of the CrossBoss entering a side branch, it should be withdrawn and a guidewire used to track beyond the side branch ostium, allowing the CrossBoss to then be delivered beyond this (wire redirect).80 In the event that wire crossing beyond the side branch is not possible, a knuckled wire can be used to cross the side branch then redeliver the CrossBoss and attempt advancement once again (knuckle redirect).80 Should this also not be successful, a small balloon with a 1:1 ratio to the side branch can be placed in the side branch ostium to deflect passage of a knuckled guidewire into the side branch subintimal space or, alternatively, a dual lumen catheter can be used to allow a second guidewire to cross beyond the side branch, thereby enabling advancement of the CrossBoss also beyond the side branch ostium, where it can then be advanced ahead of the guidewire.80
Adjunctive Equipment
Adequate support is key to overcoming the proximal and distal caps. This comes initially from the choice of vascular access. Femoral access affords larger bore access (up to 8 Fr commonly used) with the option of long sheaths to overcome iliac and aortic tortuosity. Biradial access may also allow insertion of a 7 Fr sheath, particularly when using slender sheaths that reduce the outer diameter by 1 Fr. Therefore, appropriate guide catheter selection is imperative, and guide catheters should be selected specifically for graft access where retrograde approaches or distal landing zone visualisation is necessary. Adjunctive support systems, such as guide catheter extensions, provide additional support to aid cap puncture, but are particularly beneficial during ADR.
Advancement of the guide catheter extension into the coronary artery to the point at which endothelial dissection occurred can reduce influx of blood into the subintimal space, thereby reducing haematoma formation and subsequent compression of the true lumen. Re-entry beyond the distal cap is therefore aided using a guide catheter extension, maintaining luminal size for a greater likelihood of success into the true lumen. Post-CABG CTO crossing may require multiple wire changes in AWE or ADR approaches, particularly when using knuckle wires or the CrossBoss catheter; as such, equipment allowing rapid exchange with balloon trapping aids efficiency. The Trapliner (Teleflex) guide extension catheter features a proximal balloon that aids this without the need for additional balloon trapping within the guide catheter system and can be a useful tool in cases such as post-CABG CTO revascularisation.
In longer CTOs, a retrograde approach using both retrograde dissection re-entry (RDR) and ADR techniques may be necessary.78,97,98 RDR will involve accessing the subintimal space either distal to or through the distal CTO cap, and the reverse controlled antegrade and retrograde tracking (reverse CART) technique is currently the dominant RDR technique, with high success rates.117 The antegrade aspect to reverse CART involves ADR to facilitate overlapping knuckle wires followed by balloon dilation of the subintimal space to connect the common space between the retrograde and antegrade dissection planes. Following this, retrograde wiring of the guide catheter can be performed should the reverse CART be performed in the proximal portion of the vessel or if more distal a guide catheter extension can be advanced to the point of antegrade dissection (as described above) and facilitate efficient retrograde wiring of the antegrade guide.
Microcatheters have greatly improved the efficiency of wire exchange but also provide additive penetrative forces that can be applied to high-resistance areas within the CTO. Each microcatheter retains specific properties that allow engagement into the proximal cap and can provide support for microcatheter and wire advancement with exchange when necessary. This can reduce friction on the wire through the body of the CTO and allow improved torque transmission.79 Microcatheters vary in their construction and so possess specific properties in terms of their size, lubricity, push force and the ability to track the wire and vessel. Microcatheters can be categorised as coil and non-coil based, with braided and non-braided catheters suited to different levels of penetration force and anatomy. Further details and comparisons of selected microcatheters can be found in the antegrade CTO book by Spratt et al. (chapter 7, section 20).79
As described above, calcium is a prominent feature in post-CABG patients. As such, it is essential to have calcium modification and imaging tools available and to use them where necessary. Rotational atherectomy (‘rotablation’) and, more recently, intravascular lithotripsy (IVL; Shockwave Medical, Fremont, California) provide tools to modify calcium, whereas intravascular imaging tools, such as intravascular ultrasound, are critical tools required to understand the calcium burden, pattern and location and the interval effects of calcium modification.118–120 Other available calcium-modification tools include cutting, scoring and high-pressure balloons. It may be necessary to use these tools in conjunction with each other to allow successful CTO crossing and optimal stent placement in CTO vessels with a high calcium burden.
Deliberate Vein Graft Sacrifice
Following successful revascularisation of CTOs in post-CABG patients, consideration should be given as to whether a patent SVG will provide excessive competitive flow to the distal vessel and therewith reduce long-term patency rates in the reconstructed native vessel. In a retrospective analysis of consecutive post-CABG patients where deliberate SVG sacrifice was performed, mostly by using vascular plugs, Wilson et al. demonstrated this to be a safe and effective method, with high success and low periprocedural complication rates, in these patients.121 Although more data are still needed to demonstrate whether this technique can improve long-term revascularised CTO vessel patency, consideration should be given to this approach in selected cases.
Conclusion
CTO crossing has improved with available data, advances in technology and techniques, among which the hybrid algorithm has played a crucial role, resulting in high success rates and, importantly, excellent long-term outcomes. Understanding the challenges of CTO revascularisation in post-CABG patients in terms of anatomical and lesion characteristics and clinical patient factors is necessary to prepare operators for selecting appropriate strategies and techniques that it may be necessary to have available in the operator armamentarium for successful CTO crossing and outcomes. Older, frailer patients with multiple comorbidities and more complex, established lesions with increased anatomical variance will need to be appreciated and contended with. Antegrade CTO crossing in these patients is possible, yet it is important to recognise the need to have retrograde options available, particularly because vein grafts can act as excellent conduits to the distal vessel. Experienced operators and high-volume centres will offer these patients a good chance of improvements in symptoms and quality of life, the essence of CTO treatment.