Out-of-hospital cardiac arrest (OHCA) is a significant challenge for the National Health Service (NHS). In England in 2019, ambulance services responded to over 80,000 cardiac arrest calls, of which 31,146 subsequently received treatment.1 The incidence of cardiac arrest was 56.5 per 100,000, with a median age 70.4 years. Fewer than one-third (30.7%) of patients in whom resuscitation was attempted by ambulance staff were admitted to hospital with return of spontaneous circulation (ROSC), and overall survival to hospital discharge was 9.6%, which compares unfavourably with other countries in Europe.1,2
Each nation of the UK has identified OHCA as a priority condition and published a cardiac arrest strategy to address this. In 2017, a national framework for OHCA in England, Resuscitation to Recovery, set out recommendations across the patient pathway (‘chain of survival’) to improve outcomes.3 One key recommendation was that all patients with ROSC should be taken to a designated cardiac arrest centre (CAC) for further assessment, triage and appropriate treatment. These recommendations were endorsed by 20 professional associations, including the British Cardiovascular Intervention Society (BCIS), and supported by a further five, including the National Institute for Cardiovascular Outcomes Research and the National Audit of Cardiac Rehabilitation. Furthermore, the National Confidential Enquiry into Patient Outcome and Death review of hospital care of patients admitted after OHCA recently identified a number of areas for improvement, including prompt access to cardiologists and interventional cardiology services.4
Recommendations for the establishment of regional CACs have also been published by the American Heart Association, as well as in a recent position paper from the Association for Acute Cardiovascular Care of the European Society of Cardiology in conjunction with several other European societies.5,6
Aims and Objectives
Despite the plethora of recommendations, regional CACs in England have not been widely adopted on a formal basis. Based on mounting evidence of the benefit to patient care and the enormous variability in UK clinical practice in relation to treatment of OHCA in general, and by interventional cardiologists in particular, where provision of emergency coronary angiography (CAG) varies from 0.09% to 4.74%, it is broadly accepted that it is time to re-establish cardiac networks in order to coordinate the planning and delivery of systems to improve the quality of care and outcomes for these patients.7 The BCIS OHCA focus group recognises that there remains significant heterogeneity of services offered by current CACs, ranging from standalone cardiac specialist centres to others that are based within larger hospitals with access to a range of other specialities. It is also acknowledged that there is a lack of high-quality evidence to support wide-scale changes in pathways of care, but that interpretation of the current evidence base, together with a pragmatic standardisation of practice, is needed to reduce this variability and provide high-quality care. The recommendations made here apply to interventional cardiology services in particular, but inevitably overlap with other specialties, reflecting the complexity and multisystem manifestation of this condition.
Accordingly, three key recommendations underpin this proposal:
- formal establishment of cardiac arrest centres with clear terms of reference for specialist cardiac service provision;
- development of a pathway of care for the selection and conveyance of comatose OHCA patients to these centres (because the non-comatose OHCA survivor has survival similar to the non-arrested acute coronary syndrome patient and should be treated in accordance with these established pathways);8,9 and
- development of a standardised protocol for the initial assessment and cardiovascular management of the OHCA patient at the CAC.
Establishment of Dedicated Cardiac Arrest Centres
Rationale
The rationale for regionalisation of care for patients with OHCA in dedicated CACs is based on the potential for early provision of specialist care pathways, including cardiovascular investigations and therapies, intensive care expertise and rehabilitation.6 It is known that regionalisation of care to specialist centres is of benefit in other acute conditions, such as trauma, stroke and ST-segment elevation MI (STEMI).10–12 Evidence supporting regionalised care for OHCA is largely based on international studies, which demonstrate variable effect sizes associated with this change in practice.13 These observational registries suggest that admission to high-volume centres, particularly those with access to 24/7 primary percutaneous coronary intervention (PCI) facilities, is associated with optimal provision of cardiovascular investigations, critical care and improved outcome.14–16
Evidence Base
The applicability of this evidence is potentially limited by differences in systems of care where rates of bystander cardiopulmonary resuscitation (CPR) can vary, geographic variation with different journey times in the UK and the clear limitations of observational evidence, which is at risk of selection bias. For example, observational data from one study in the UK suggest that direct admission to a dedicated heart attack centre is associated with higher provision of invasive coronary angiography (CAG) but, in this study, was not associated with improved survival.17 There remains significant variation in outcome across the UK; an analysis of 17,604 patients admitted after an OHCA to 239 hospitals in England and Wales identified substantial variation, whereby mortality by hospital discharge ranged from 10.7% to 66.3% (median 28.6%; interquartile range [IQR] 23.2–39.1%), with patient and health service factors explaining only 36.1% of this variation.18 This outcome difference may be explained, at least in part, by the obvious variability in interventional cardiology practice.7 In a recent large multicentre observational that included data from three ambulance services in England for >10,000 patients, covering approximately one-third of the country’s population over a representative geographic area, direct admission to a CAC with 24/7 primary PCI availability was associated with an absolute improvement in survival to hospital discharge of 2.5% in all OHCA patients (OR 1.69; 95% CI [1.28–2.23]).19
Proposal
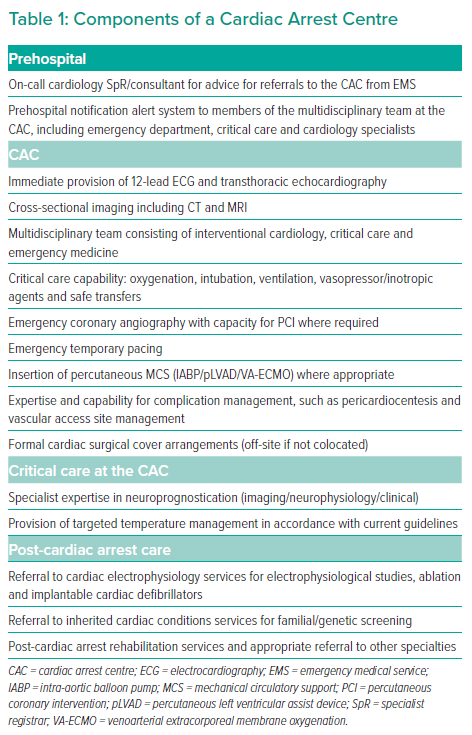
The current evidence indicates the potential importance of standardisation of care for OHCA in the UK to reduce heterogeneity in practice, and an essential component to achieve this aim would be formalisation of a network of dedicated CACs. The components of our definition of a CAC are summarised in Table 1. Briefly, these centres should be able to provide a range of 24/7 services, including emergency CAG and PCI, specialist cardiovascular and cross-sectional imaging, intensive care expertise and multimodal neuroprognostication. It is acknowledged that certain CACs will also provide specialist services for cardiogenic shock, such as mechanical circulatory support, extracorporeal membranous oxygenation (ECMO) and extracorporeal CPR (ECPR). Different models for nationwide provision have been proposed, including a hub-and-spoke model, and the BCIS will establish a separate focus group to address this challenge. The establishment of CACs also provides a unique opportunity to address inadequacies in post-discharge care by developing tailored rehabilitation services addressing physical, neurological and psychosocial needs.20 It is envisaged that the dedicated CACs will generally be modelled on existing primary PCI centres and there is therefore the realistic potential for several centres within a region to be able to provide the necessary services and be designated as a formal CAC. However, the CAC structure depends on the concept that it is led in each hospital by a core team of clinical champions in each stakeholder specialty, including interventional cardiology, critical care/anaesthetics and emergency department physicians (Table 1).
Proposed Pathway of Care for Conveyance of Patients to Cardiac Arrest Centres
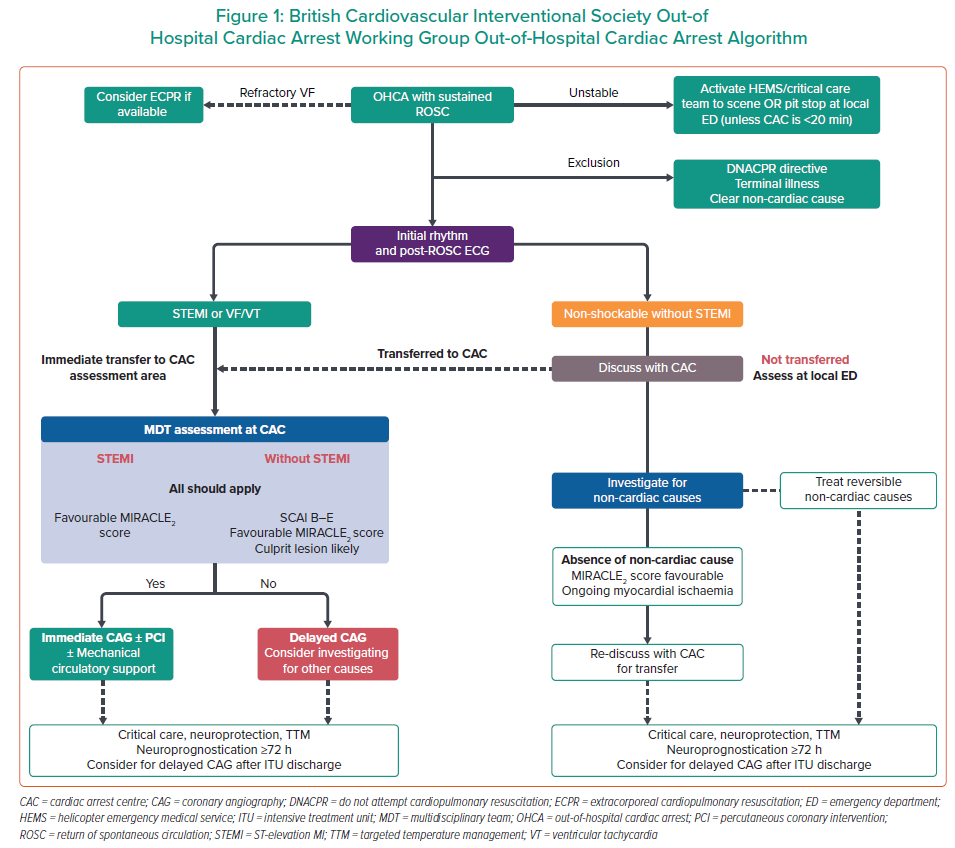
Based on the available evidence and expert consensus, we suggest a post-resuscitated cardiac arrest pathway that includes readily available information from the prehospital scene to ensure that patients who will likely benefit most from the range of services provided in a CAC, particularly the cardiovascular aspects of this care, are transferred without delay. This pathway is outlined in Figure 1 and further described below.
Although several organisations and consensus groups now recommend that all patients with OHCA are conveyed directly to a CAC, this has the potential for significant financial and logistical burden in cases for which it is not justifiable, so we currently favour a more selective, tailored approach.21 Furthermore, the current evidence does not support conveyance of the entire population of OHCA. Specifically, although it is generally accepted that all patients with STEMI on 12-lead ECG should be conveyed for an emergency assessment and subsequent primary PCI, the data are less clear for those without STEMI. Given that these cases are the most common, the debate about their best treatment is important: the ambition to produce neurologically intact survivors must be tempered against the emotional and financial costs of intensive therapies in cases that are futile either from a survival point of view or by virtue of profound hypoxic brain injury.22 The ARREST trial is currently comparing direct conveyance to a CAC compared with standard of care to emergency departments in OHCA patients without STEMI and is projected to report its findings by the end of 2023.23
Increasingly, current evidence indicates that the selection of patients with characteristics that increase the probability of a cardiovascular cause may identify a group that benefits most from direct conveyance to a CAC. Patients with STEMI on 12-lead ECG have a high risk of a culprit lesion and, despite the lack of evidence from randomised controlled trials (RCTs), the recommendation is for direct conveyance to a CAC.24 However, several studies also suggest that patients presenting with a shockable rhythm or pulseless electrical activity (PEA) derive most benefit from this approach. A subgroup analysis from the study of von Vopelius-Feldt et al. indicated that survival benefit was mainly seen in patients with shockable initial rhythms or a first recorded rhythm of PEA.19 However, that study did not demonstrate a clear benefit for patients with asystole on ambulance arrival or without sustained prehospital ROSC. If the analysis is restricted only to cases of OHCA due to either PEA or a shockable rhythm and sustained ROSC, the potential benefit of admission to a CAC increases to 4.4% (OR 1.58; 95% CI [1.15–2.17]).19 This would correspond to a number needed to treat (NNT) of 23, which is comparable to other common acute cardiac interventions.25 This is further corroborated by data from Arizona (US), which showed that state-wide regionalisation of care in CACs with access to 24/7 primary PCI improved neurological outcome at hospital discharge compared with historical controls, but that this was most marked in those with an initial shockable rhythm.26
Prehospital Treatment of Out-of-Hospital Cardiac Arrest and Links to Cardiac Arrest Centres
For a cardiac arrest system to deliver improved outcomes, optimal case selection starts in the prehospital phase, where rapid, effective resuscitation according to current guidelines remains the foundation of successful outcomes.27 The Joint Royal College Ambulance Liaison Committee guidelines have previously outlined the key components of post-resuscitation care in the field.28 Where ROSC has been achieved, a rapid primary survey assessment should follow, with an early ECG to detect overt evidence of STEMI with a view to immediate transfer. If airway protection or cerebral agitation is a concern, early mobilisation of a critical care team to the scene to provide airway support can be considered to facilitate transfer direct to a CAC. Where this is not possible, transfer to the nearest emergency department may be needed for stabilisation, but this should ideally be avoided to prevent inevitable delays to definitive treatment.
It is recommended that these systems deploy staff with appropriate experience in this condition, such as advanced paramedic practitioners and/or prehospital physicians to lead the cardiac arrest and subsequent transfers team where possible.29–31 As detailed below, we advocate clear pathways for immediate transfer to a CAC for selected patients, but equally propose that established lines of communication with the CAC are maintained for discussion of borderline cases where immediate transfer is currently not mandated. Secondary transfer (i.e. admission to one hospital for initial assessment and then requiring a further ambulance journey) is to be avoided wherever possible. However, additional diagnostics at a receiving non-CAC may yield information that increases the likelihood of benefit of treatment at a CAC and, in these circumstances, a secondary referral should be made to the CAC in a similar standardised fashion as for prehospital referrals.
Prehospital Case Selection for Conveyance to Cardiac Arrest Centres
When considering patient selection for direct admission to a CAC from the prehospital scene, we propose to divide patients with OHCA into three main groups: patients without ROSC; patients requiring urgent transfer to a CAC; and patients not requiring urgent transfer to a CAC.
Patients Without Return of Spontaneous Circulation
Currently, patients without ROSC have a very poor prognosis and we do not recommend routine transfer to a CAC. However, the future for such patients may well be more positive as evidence for novel therapies emerges. Initial observational studies have demonstrated the ineffectiveness of CPR during ambulance transfer.32 In addition, it has been suggested that direct admission to a CAC, with resulting longer transfer times, may not be beneficial in this patient group.19,33 The ARREST study, from Minnesota, indicated that hyperinvasive protocols that incorporate immediate ECPR on arrival to a hospital in combination with early angiography can be beneficial.34 This is supported by observational data from Paris, where there is broad experience for application of ECPR in a prehospital setting by specialist emergency response teams.35 However, it should be noted that the recent Prague OHCA trial did not show that hyperinvasive protocols that incorporate ECPR significantly improve survival with a favourable neurological outcome at 180 days compared with standard resuscitation.36 Furthermore, there are important obstacles to the wide-scale provision of ECPR that must be considered, including a proposed 60-minute time frame from arrest to initiation of venoarterial ECMO, the requirement for mature local networks and interdepartmental pathways, understanding optimal patient selection and overcoming the logistical and financial implications of delivering such pathways of care. Networks of cardiovascular care in the UK are not established to provide this service currently, but a prehospital feasibility study is under way in London and, should this be positive, may lead to further clinical application.37 Importantly, it is acknowledged that these pathways must be closely linked with the facilities available in dedicated CACs and provide further justification for their establishment.
Patients Requiring Urgent Transfer to a Cardiac Arrest Centre
Patients requiring urgent transfer to a CAC include those with ST elevation on either pre-arrest or post-ROSC ECG and those with an initial shockable rhythm regardless of admission 12-lead ECG findings.
Patients With ST-elevation MI
There is general consensus, and moderate evidence, that patients with STEMI on 12-lead ECG benefit from immediate primary PCI at a CAC, which is reflected in current European Society of Cardiology, European Association for Percutaneous Cardiovascular Interventions and European Resuscitation Council (ERC) guidance, although it is acknowledged that there is no RCT evidence for this recommendation.5,18,25 The consensus is, of course, driven by the unequivocal benefit achieved by primary PCI in STEMI patients as a whole.
Patients Without ST-elevation MI and Initial Shockable Rhythm
The immediate transfer of patients without STEMI for the provision of early CAG is controversial. In previous observational registries, the rates of culprit lesions in this patient group varied from 20% to 50%, with some evidence of an association of early CAG with improvement in survival.38 However, three recent RCTs (COACT, PEARL and TOMAHAWK) have shown no benefit from early versus delayed angiography in this patient group.39–41 These results have been further corroborated by the EMERGE trial.42 This may indicate that for certain patients in this cohort a delayed invasive approach may, indeed, be appropriate. However, the findings of these RCTs should be interpreted with some caution, which prevents generalisation to all patients without STEMI in clinical practice. The key exclusion criteria of COACT and TOMAHAWK were patients with STEMI, unstable haemodynamics, a non-cardiac cause of arrest and (in COACT) those with a non-shockable rhythm and severe renal disease. Data from the EUCAR Registry indicate that these exclusion criteria limit the external applicability of both these studies to the real-world OHCA populations without STEMI.43 It is also acknowledged that an important disadvantage of RCTs is that by their design they will only, by chance, enable detection of whether particular subgroups may derive benefit from a strategy. Second, all patients in these trials received care at dedicated CACs and there were high rates of coronary artery disease on CAG (approximately 50–70%), with <30% requiring acute revascularisation. Two-thirds of patients in the delayed arm also had CAG at a median time frame of 2–5 days, with <15% requiring immediate crossover for urgent indications, highlighting the importance of being situated in a CAC.39–41 Patients with an initial shockable rhythm, regardless of 12-lead ECG findings, have high rates of CAD or other cardiac aetiologies and have been identified as a group with the potential for most benefit at a CAC.19,44
Accordingly, we recommend that patients at the highest risk of cardiac aetiology OHCA may be best conveyed to CACs even if they do not receive immediate CAG so that rescue coronary angiography can be provided on a 24/7 basis or mechanical circulatory support (MCS) can be provided in a timely fashion. In this phase of the guidance, this should include patients with STEMI on post-ROSC ECG or those with an initial shockable rhythm. It is widely accepted that intensive therapy units (ITUs) at CACs will have greater experience and expertise in managing the post-OHCA patient. Although immediate CAG on arrival itself is not mandated, it is recommended that patients receive, as a minimum, prompt assessment and treatment by a multidisciplinary team of specialists; observational research supports that this subgroup of patients is likely to benefit from this approach.13,19
Patients Not Requiring Urgent Transfer to a Cardiac Arrest Centre
Patients not requiring urgent transfer to a CAC includes those with sustained ROSC with OHCA due to PEA or asystole without clear signs of STEMI on 12-lead ECG. This represents an extremely heterogeneous group of patients, with cardiac arrest potentially due to a variety of pathophysiology, ranging from intracranial or abdominal vascular catastrophe to toxicology, sepsis or respiratory failure, frequently with underlying chronic illness or frailty.45,46 Recent RCT evidence indicating that a delayed invasive approach is an appropriate strategy in selected patients without STEMI suggests that there is adequate time for stabilisation and to perform a complete diagnostic evaluation in this setting.39–41 Patient conveyance from the scene to either a CAC or the nearest hospital should therefore be a tailored, patient-specific decision, based on the best available information at the time. Communication can be made via established and standardised pathways to allow reliable discussion with a nominated point of contact within the receiving CAC. Importantly, this information may change with further diagnostics at the receiving hospital, and it is recommended that further contact be made in this case.
Protocol for Initial Assessment and Management of the Out-of-Hospital Cardiac Arrest Patient at the Cardiac Arrest Centre
Location of Assessment and Triage at the Cardiac Arrest Centre
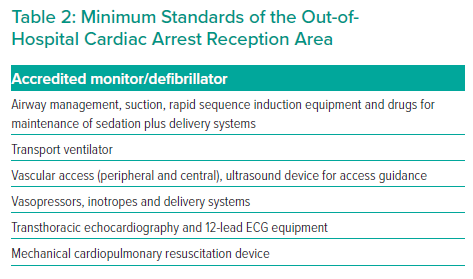
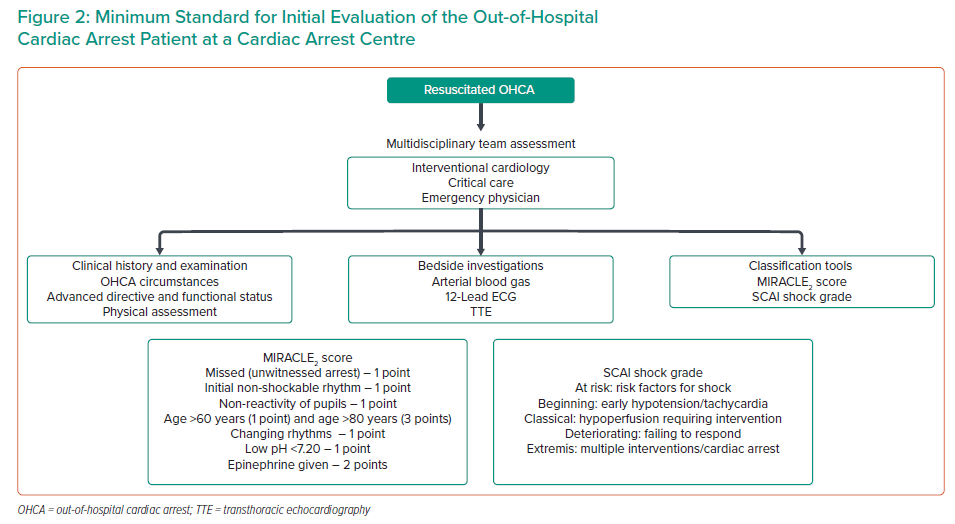
The location for initial assessment at the CAC will vary between regionalised systems of care and should define a suitable stabilisation area for the reception and initial assessment of OHCA cases. Whether this is based in the cardiac cath lab or within the emergency department, it should be equipped with fully serviced resuscitation and critical care equipment suitable for the ongoing care of ventilated and/or shocked patients. A process should be developed for a pre-alert notification system for the multidisciplinary team consisting of emergency department, critical care and cardiology specialists, to enable safe and efficient handover of care between pre- and in-hospital teams. A minimum standard for the composition of the OHCA reception area and for the initial evaluation of a patient on arrival to a CAC are shown in Figure 2 and Table 2.
Specific Considerations and Provision of Cardiac Arrest Centre Treatment
Twelve-lead ECG and transthoracic echocardiography (TTE) form the fundamental basis of urgent assessment at a CAC. The recent PEACE study showed that when 12-lead ECGs are performed at a later time point after OHCA, such as on arrival to a centre, their diagnostic accuracy increases.47 Nevertheless, the 12-lead ECG alone can be a poor predictor of a culprit lesion and excluding significant CAD by means of urgent invasive coronary angiography may, in itself, be beneficial for refocusing ongoing care. In addition, TTE is an essential component of early assessment to understand left ventricular systolic function, regional wall motion abnormalities and mechanical complications of MI. It may also uncover non-coronary causes, such as aortic dissection, cardiac tamponade and pulmonary embolism. Recent data suggest that the presence of a regional wall motion abnormality on arrival to a heart attack centre is associated with substantially higher rates of culprit coronary artery lesions and may guide patient selection for invasive coronary angiography.48 Thus, the immediate availability of TTE is viewed as a cornerstone of the emergency cardiological assessment of OHCA patients.
As discussed above, three landmark RCTs have recently shown that an early invasive approach is non-superior to a delayed approach in patients without STEMI.39–41 It may be reasonable to withhold an immediate invasive approach in patients who meet the restricted selection criteria of these RCTs and in whom a non-cardiac aetiology is suspected. However, it is important to note that the RCT study criteria are not generalisable to unselected cases of OHCA in a real-world setting. Importantly, myocardial ischaemia and haemodynamic instability were not specifically included in these studies where invasive CAG is likely to be of substantial benefit. In a real-world setting, it is also acknowledged that there is no clinical disadvantage for an early strategy, and performing immediate PCI and definitive treatment of reversible causes may provide confidence to implement earlier extubation, leading to reduced ITU and hospital stays. However, current data evaluating this strategy are limited, and it requires more formal study.49 A finding of unobstructed coronary arteries can also be of use in understanding the aetiology of the OHCA even though it cannot exclude all ischaemic aetiologies, such as coronary vasospasm or embolic disease. Hence, it is recommended that clinical discretion is used when considering immediate invasive coronary angiography, particularly in patients without STEMI.
Methods to Help Assess Suitability for Angiography and Intervention: MIRACLE2 Score
Mortality after OHCA remains high due to irreversible neurological injury, which accounts for >60% of deaths in patients admitted after ROSC.22,25 Hence, it is increasingly appreciated that an attempt at understanding the unfavourability of the cardiac arrest circumstances should be incorporated into the decision-making process to ensure that those with a chance of good outcome are not denied optimal care while also avoiding the expenditure of limited resources in cases of clear futility.50,51 The importance of objective estimation of risk of poor outcome prior to delivering invasive therapies is recognised and, where possible, should incorporate risk tools as opposed to subjective perceptions of risk or factors in isolation such as ‘downtime’.
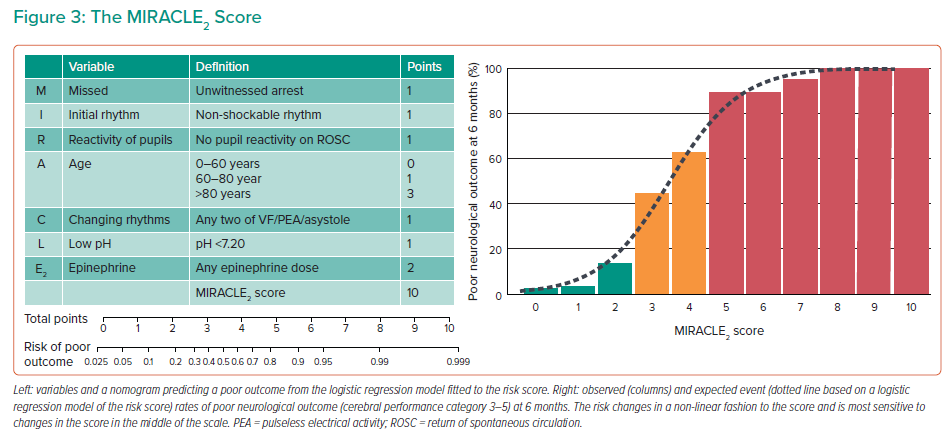
The MIRACLE2 score was recently developed and validated in 846 patients specifically for use on arrival to a CAC with high accuracy for the prediction of poor outcome (area under the curve 0.84–0.91).52 Unlike other models, MIRACLE2 is simple to use: it incorporates seven variables that are all readily available on admission or prior to conveyance to a CAC with a total possible score of 10 points; increasing scores predict poorer outcome (Figure 3). Patients can be classified into low (MIRACLE2 ≤2), intermediate (MIRACLE2 3–4), high (MIRACLE2 ≥5) and very high (MIRACLE2 ≥7) risk, with risks of poor outcome in these groups being 5.6%, 55.4%, 92.3% and 99.5%, respectively. Importantly, the MIRACLE2 score predicts poor neurological outcome at 6 months rather than mortality, which is a more clinically relevant outcome and ensures that patients with late recovery are taken into account.53 It is recommended that the MIRACLE2 score is used as an objective risk tool to specifically identify those with an appropriate chance of survival to ensure that full active treatment is appropriately provided. Hence, we recommend that MIRACLE2 should be used as an adjunct to decision making in these cases.
Cardiogenic shock occurs in over half of OHCA patients and is associated with increased severity of CAD and worse clinical outcomes.54 There is high-level evidence from the SHOCK and CULPRIT-SHOCK trials that revascularisation of the culprit lesion is of benefit in this patient group.55,56 Patients with cardiogenic shock were excluded from the recently reported RCTs, and the presence of haemodynamic instability is known to be associated with increased severity of CAD.55,56 Several MCS devices are now available that unequivocally improve haemodynamics, but have nevertheless failed to show benefit in clinical outcomes in RCTs to date.57,58 As further studies are undertaken and evidence emerges, a role for MCS may emerge in some of these patients. Owing to the lack of supportive clinical data to date, patients treated with advanced MCS should have this provided only as part of registries or systematic clinical trials where possible. The SCAI shock grade was recently developed as a practical assessment to standardise the classification of shock and guide therapeutic approaches.59 We recommend that the SCAI grading system should be incorporated into the initial assessment to guide treatment strategy and that the presence of haemodynamic instability should be viewed as a strong clinical indication for the performance of invasive coronary angiography after OHCA.
Summary of Decision for an Early Invasive Approach
It is recommended that a joint decision for an early invasive approach is made by a multidisciplinary team, consisting of trained interventional cardiologists and critical care and emergency department physicians, on arrival to a CAC. This decision should incorporate a thorough specialist and objective assessment of ECG and echocardiographic findings, haemodynamic instability (SCAI shock grade) and clinical appropriateness in terms of clinical state and the absence of futility by application of the MIRACLE2 score. Although the threshold will vary in different clinical situations, in general, patients with a MIRACLE2 score of 0–4 are identified as being at low risk and can be considered for full active care, whereas those with a score ≥7 are at very high risk of futility, and invasive therapies such as CAG and MCS, particularly in those without STEMI, may not be immediately appropriate. A combined personalised approach to early invasive CAG is supported by recent evidence from EUCAR, which suggests that an early invasive approach may continue to be of benefit in those with a low MIRACLE2 score, either with STEMI or with a SCAI shock grade of B–E.60 These data are observational and hypothesis generating, so require prospective validation prior to exact thresholds being selected for the performance of early invasive CAG.
In summary, we recommend that, in the presence of STEMI on admission 12-lead ECG, an immediate invasive approach should be considered in all patients after assessment of the favourability of the OHCA circumstances in accordance with current guidelines.25,51,61 For patients without STEMI, we continue to recommend an early invasive approach with potential-culprit-vessel-only PCI in those with haemodynamic instability, favourable cardiac arrest circumstances and a high likelihood of clinically significant CAD.56 Patients deemed not appropriate for an initial early invasive approach can be stabilised and evaluated for other causes while receiving supportive care and awaiting neurological recovery. However, this group should be monitored carefully in the event that they develop haemodynamic instability or ischaemia and require rescue PCI.
Estimated Impact of the Suggested Pathway on Current Practice
A major barrier to the implementation of regionalisation of OHCA care in CACs is the concern about increased resource use.19 The likely increase in workload that CACs could expect as the result of the proposed pathway was estimated at an additional 35–48 cases of OHCA per CAC per year. Within each CAC, the impact on the emergency department is likely negligible, given that most emergency departments see between 50,000 and 150,000 patients per year.4 From an interventional cardiology perspective, the BCIS recommendations state that primary PCI for STEMI should be undertaken in hospitals that perform >300 primary PCIs per year.62 Bypass of OHCA patients to CACs will include primary PCI for most of these patients, which may help lower-volume CACs to achieve the minimum recommended numbers and increase the workload by <10% for other CACs. Finally, many of these patients will be admitted to an ITU. A recent analysis of national UK intensive care data showed a median length of ITU stay of 2.7 days (IQR 1.0–5.9 days), resulting in an estimated additional 110 ITU days (IQR 40–236 ITU days) at each CAC.63 Of note, the additional workload is likely to vary considerably between individual CACs and some patients already currently undergo secondary transfer to a CAC ITU for ongoing care after initial stabilisation. There is evidence of ambulance crews already bypassing local hospitals in favour of CACs in urban areas with short transport times and these CACs will see little change compared with CACs in more rural areas.19 Finally, it is possible that direct admission to an ITU with specialist expertise may enable earlier neuroprognostication, leading to a shorter length of stay than in a non-CAC ITU. Supplementary Table 1 summarises the estimated impact of the new pathway, based on recent data from English ambulance services.19
Gaps in the Evidence
Prehospital
Further RCTs are required to understand the role of ECPR in refractory cardiac arrest and how these can be linked with specialist CACs.
RCT data are required to understand whether direct conveyance to a CAC is beneficial for patients with OHCA without STEMI.
The role of prehospital stratification in patients with resuscitated OHCA, and whether this may guide appropriate conveyance to a CAC, requires clarification.
Post-hospital
Further RCTs are required to understand the role of early invasive angiography in patients with OHCA both with and without STEMI on 12-lead ECG. RCT data are required to understand the role of advanced MCS for patients with OHCA and cardiogenic shock, but such RCTs would be challenging to perform.
Further studies are required to understand whether risk stratification based on subgroups of OHCA may guide the selection of therapies, such as early invasive angiography or neuroprotective therapies.
Summary and Vision
Five years since the publication of Resuscitation to Recovery, little progress has been made in the field of post-OHCA care in England.3 Our aim is to reinvigorate the desire to improve patient care through the presentation of an OHCA care pathway that combines the best available evidence, pragmatism and clear direction or flexibility as required. In particular, we wish to eliminate unwarranted variation in practice that is currently manifest in the UK, which will lead to the equitable provision of cardiovascular therapies, specialist critical care input and post-discharge rehabilitation.
Our guidance shares several similarities with that of the American Heart Association and ERC, which includes the establishment of regional CACs with the provision of specialist facilities, including ITU, targeted temperature management and advanced neuroprognostication, where appropriate. There is agreement that all STEMI patients ought to be offered immediate CAG and non-STEMI patients should be evaluated on a case-by-case basis. Although it is appreciated that this pathway represents a departure from the original notion that CAC admission should be offered across the board after OHCA, we believe that the inclusion of more recent evidence published since 2017 will aid regionalisation of OHCA care in a phased manner. Now is the time to re-establish cardiac networks and to coordinate the planning and delivery of systems with the primary goal of standardising and enhancing the quality of care and clinical outcomes for our sickest patients across the UK.