Aortic stenosis (AS) is one of the most common valvular heart diseases and its prevalence increases with age. In the elderly, the prevalence of severe AS is up to 3–5%.1,2 Coronary artery disease (CAD) is common in this cohort because of shared risk factors and pathophysiology.3
Treatment of severe aortic stenosis with CAD has traditionally been with surgical aortic valve replacement (SAVR) plus coronary artery bypass grafting (CABG).4,5 With the advent of transcatheter aortic valve implantation (TAVI), the option of TAVI plus percutaneous coronary intervention (PCI) is another viable alternative for such patients.6
Assessment and management of CAD in this setting involve unique challenges and considerations because of complex physiological and anatomical interactions involved in aortic stenosis, the transcatheter heart valve (THV), as well as the TAVI procedure itself.7
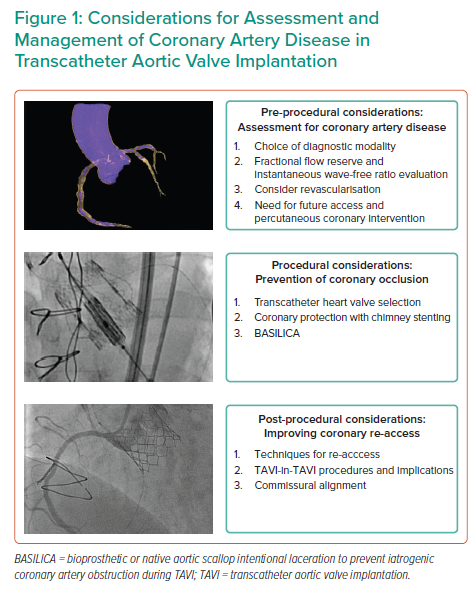
This review will outline the epidemiology and pathophysiology of CAD in patients undergoing TAVI; describe the pre-procedural assessment and management of CAD; describe procedural concerns and techniques to avoid coronary obstruction; and describe post-procedural coronary re-access strategies (Figure 1).
Pathophysiology and Prevalence
Degeneration of the aortic valve is the most common cause of AS, followed by bicuspid aortic valve and rheumatic degeneration. Degeneration is associated not only with age but also with dynamic inflammation, lipid accumulation and subsequent calcification.8 These processes are related to risk factors of atherosclerosis. Hypertension, diabetes and dyslipidaemia have been found to have dose-response associations with the onset of severe AS, whereas other risk factors that have been implicated include age, tobacco consumption and waist circumference.8–11 Patients with CAD share these risk factors, which explains the close association between the two conditions.
In comparison, bicuspid aortic valves exhibit degenerative calcification primarily because of mechanical stress. Studies have shown that in patients with bicuspid aortic valves, aortic sclerosis starts in the second decade while calcification is seen in the fourth decade of life. Symptom onset is thus earlier at ages 50–60 years.12–14 In a meta-analysis that included 31 studies with a total of 7,603 subjects, patients with bicuspid aortic valve were compared with those with degenerative aortic valve disease; the former group was younger (by a mean 7.29 years; 95% CI [11.17–3.41]) with fewer comorbidities and had only one-third the prevalence (OR 0.33; 95% CI [0.17–0.65]) of CAD.15
On histology, stenotic valves exhibit lipid deposition as well as macrophage and T-cell infiltration consistent with inflammation. Heterotopic ossification occurs, including mature lamellar bone formation and active bone remodelling, accounting for calcification as a common endpoint of degenerated valves regardless of inciting pathology.16–18
CAD and severe AS both increase in prevalence with age, resulting in a significant overlap in patient populations. The proportion of patients with aortic stenosis with significant CAD has been estimated to be between 24% and 45%. The presence of typical angina may predict a higher likelihood of CAD.19–21
In patients with severe AS undergoing TAVI, the prevalence of CAD is influenced by their risk profile and demographics. Initial trials of both self-expanding and balloon-expandable THVs were conducted in prohibitive or high-risk patients. These patients were elderly and had multiple comorbidities including diabetes, hypertension and dyslipidaemia. In such a population, the prevalence of CAD was found to be high at over >70%.22,23 In follow-up trials involving intermediate- and low-risk patients, a younger cohort with fewer cardiovascular risk factors were recruited. In these trials, the prevalence of CAD was demonstrated to be <30%.24,25
Pre-procedural Assessment and Management of CAD
Evaluation for CAD is recommended by major guidelines before aortic valve intervention because of a high prevalence, prognostic impact and potential future difficulty with coronary access.4,26 The complexity of CAD, surgical risk and aortic root and vascular anatomy should be considered by the heart team before a decision on a combined aortic valve and coronary intervention is made. A greater complexity of CAD (left main or multivessel CAD with high SYNTAX score) or high-risk aortic root anatomy may suggest an advantage for a combined SAVR and CABG strategy over TAVI and PCI.
Coronary evaluation with invasive coronary angiography was routine initially because of the high prevalence of CAD in high-risk TAVI patients. As TAVI indications expand to include low-risk cohorts, the likelihood of CAD in such patients may reduce, so the risk:benefit ratio of an invasive procedure may change. CT coronary angiography has been shown to have adequate accuracy in ruling out significant CAD in younger patients with less coronary calcification.4,27,28 An additional advantage of CT coronary angiography is that it can be integrated with pre-TAVI routine CT evaluation. This may be performed without additional contrast, saving patients invasive evaluation and procedural risks.29
After diagnosis, the prognostic impact of CAD and role of revascularisation in TAVI patients have been unclear. Initial studies have shown differing results – likely from population heterogeneity and limited sample sizes – varying from increased mortality post-TAVI to a neutral impact.30–33
In more recent years, larger registries have offered modern, real-world evidence. UK and German TAVI registry studies, as well as a study from Toulouse University Hospital, did not suggest pre-existing CAD had any impact on mortality.34–36 However, a TAVI registry from Bern, Switzerland, published increased ischaemic events and cardiovascular mortality at 1 year in patients with CAD compared to those without (HR 1.75; 95% CI [1.06–2.89]; p=0.030), although there was no signal for increased mortality when analysed by itself (HR 1.35; 95% CI [0.85–2.15]; p=0.21).37
In an aggregation of the above mixed results, a meta-analysis of performed in 2017 studied 8013 patients undergoing TAVI with both self-expanding and balloon-expandable THVs. This showed no increase in 30-day mortality but an odds ratio of 1.21 (95% CI [1.07–1.36]; p=0.002) of all-cause mortality at 1 year.38
These mixed results are consistent with the heterogenous nature of CAD, and are likely attributable to differing inclusion criterion and degree of ischaemia present in each study (Supplementary Material Table 1). This should perhaps be unsurprising, considering background evidence in CAD in the absence of aortic stenosis, where ischaemia-guided management has been shown to be advantageous.39
When the severity of CAD was quantified with the SYNTAX score and studied, results suggested that severe CAD led to worse outcomes in TAVI patients. A SYNTAX score of >22 was more likely to be associated with increased death, stroke and MI, although another study identified a cut-off of 9 for worse outcomes.40–42 This stands in contrast with results of a recent ischaemia trial, which did not suggest benefits in revascularisation for patients with stable CAD.43 Therefore, the usefulness of SYNTAX score to guide revascularisation for patients with severe AS is still uncertain.
An objective assessment of ischaemia can be carried out through functional assessment. This may be through invasive coronary studies or with non-invasive imaging studies, such as stress echocardiography or nuclear stress tests.5,44 A major limitation of non-invasive stress testing in this cohort is haemodynamic instability, especially during exercise. This would be of particular concern in patients with symptomatic AS.
With regard to invasive functional assessment, initial concerns about the safety of intracoronary vasodilators for induction of hyperaemia have largely been allayed.45
However, physiological changes in AS such as left ventricular hypertrophy or reduction in cardiac output may impair fractional flow reserve (FFR) assessment. As a result, lesions that are not functionally significant when assessed using FFR may become significant following TAVI, although this was not reproduced in all studies.46,47
There is some evidence that instantaneous wave-free ratio (iFR) is less affected.47,48 iFR could be advantageous as coronary haemodynamics are evaluated in diastole, mitigating the effects of reduced coronary flow during systole because of the impact of severe AS on left ventricular pressures and coronary microcirculation.47 Therefore, this approach could be attractive, although more research is still required.49,50
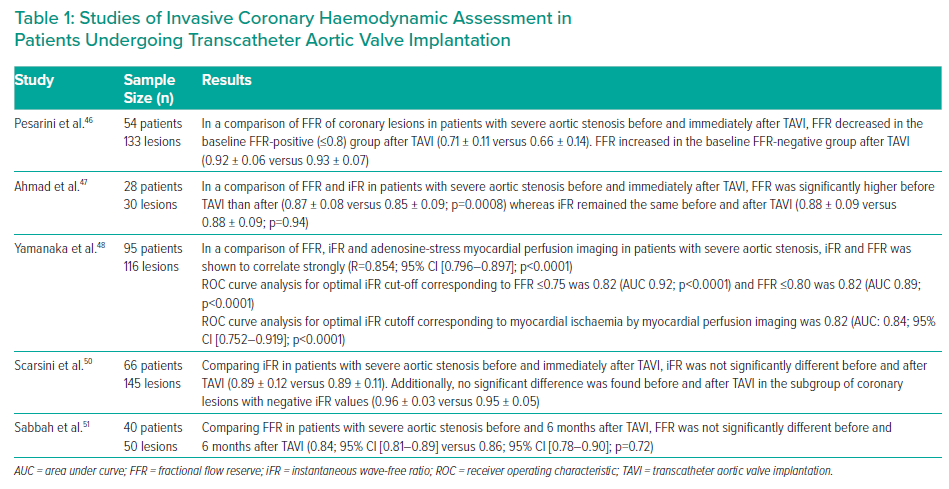
The above studies are largely short-term, periprocedural investigations, which may not account for left ventricular remodelling and unloading on a longer timespan. A recent study of FFR and resting full-cycle ratio (RFR) performed before and 6 months after TAVI suggested that FFR results were not significantly different before and after (0.84; 95% CI [0.81–0.89]; versus 0.86; (95% CI [0.78–0.90]; p=0.72), and resulted in fewer reclassifications than with a resting index, such as RFR.51 The results of studies of invasive functional assessment are summarised in Table 1.
The exact cut-offs for significant CAD in TAVI candidates are not well defined as yet. The upcoming FAITAVI trial (NCT03360591) will study the role of FFR- and iFR-guided revascularisation in such a population.
Apart from prognostic concerns, another consideration for pre-procedural revascularisation is potential difficulty with future coronary access after TAVI because of stent-frame interaction with coronary leaflets, sinuses and ostia. This is elaborated upon in the next section.
Therefore, PCI before TAVI is generally recommended for the purposes of lowering the risks of TAVI and difficult future coronary access. This is supported by major guidelines from the American College of Cardiology as well as the European Society of Cardiology, both of which recommend revascularisation of significant proximal coronary artery disease before TAVI.4,26
Although there have been historical concerns about the safety of PCI in TAVI, especially in an elderly cohort, trials and a subsequent meta-analysis have shown that PCI before TAVI does not confer any increased risk of MI, stroke, bleeding or vascular site complications.52–54 A small study of left main coronary intervention in this population (n=128 matched pairs) has also been shown to be safe in the medium term when compared against matched controls (1-year mortality 7.8 versus 8.1%; p=0.88).55
There is uncertainty over the optimal timing of revascularisation. A SURTAVI subanalysis including 128 patients suggested greater contrast use with staged PCI and TAVI compared to concomitant procedures. There is a correlation with a greater risk of acute kidney injury (11.8% versus 2.0%; p=0.04) but this is likely to be because patients with complex CAD were selected for staged PCI.56
A meta-analysis in 2017 combining the results of four studies (n=209) did not show significant differences in mortality, stroke, renal failure, MI or bleeding.57 This was supported by a subsequent observational study comparing the outcomes of 258 TAVI patients who underwent PCI (143 [55.4%] before; 77 [29.8%] concomitantly with; and 38 [14.7%] after), and did not demonstrate differences in 2-year major adverse cardiac and cerebrovascular event rates (concomitant versus pre HR 0.92; 95% CI [0.52–1.66]; p=0.79; post versus pre HR 0.45; 95% CI [0.18–1.16]; p=0.10).58
Most recently, the results of the ACTIVATION trial have been released. Of a cohort of 235 patients with severe symptomatic AS and CAD, PCI before TAVI as compared to medical management showed no difference in death, MI, stroke and acute kidney injury over a 1-year follow-up period, although there was a statistically significant increase in the risk of bleeding in the PCI group.59 Long-term follow-up data in a larger cohort will be important to evaluate this matter. Other trials comparing PCI against medical therapy before TAVI include NOTION-3 (NCT03058627) and COMPLETE-TAVR (NCT04634240). The TAVI-PCI trial (NCT04310046) aims to evaluate outcomes of patients undergoing PCI before TAVI against those undergoing PCI after TAVI.
As with CAD in general, a heart team consensus would be favourable in addressing the pre-procedural management of CAD in the setting of severe AS. Discussion points should include the need for revascularisation, as well as its timing and mode.
Procedural Considerations: Coronary Obstruction Risk and Management
Procedural coronary concerns are largely over coronary obstruction – one of the most feared complications of TAVI – which confers a high mortality rate. Fortunately, intraprocedural risk has been decreasing with improved pre-procedure imaging, patient selection and expertise as well as the availability of new-generation valves.
Occlusion can occur because of: native leaflet obstruction; sinus sequestration; obstruction of the coronary ostium by a leaflet mass; TAVI valve skirt or commissure obstruction; and deformation and stenosis of a pre-existing ostial coronary stent.60
Predisposing factors for coronary occlusion can include the patient’s anatomy and THV factors. Unfavourable anatomies include those with long leaflets (exceeding coronary ostial height), bulky leaflet calcification, low-lying coronary ostia, deficient sinus of Valsalva and low sinotubular junction height. These are routinely evaluated during pre-procedural CT imaging to identify at-risk patients. THV factors include skirt and commissural heights, the height of the valve and valve type.60,61 Procedural factors include valve deployment depth as well as valve expansion. Valve-in-valve procedures have been shown to have a higher coronary obstruction risk likely because of bioprosthetic leaflet displacement.62,63
Detailed pre-procedural analysis can reduce the risks of coronary obstruction through careful patient selection, matching the aortic root anatomy to an appropriate THV, and procedural planning to best optimise coronary visualisation and THV depth.
In those who are at a high risk of coronary obstruction despite selection and planning, there are additional techniques to mitigate this.
Coronary protection – prophylactic catheter engagement and guidewire with or without coronary balloon and stent placement in an at-risk vessel – has been long used as a protective strategy in the event of an acute obstruction.64–66 This is performed before THV deployment, as emergent attempts at cannulating coronary arteries after obstruction has occurred have a low success rate. Moreover, patients with this complication often present with dramatic haemodynamic instability rendering a salvage procedure very challenging.
Chimney stenting is a procedure where a coronary stent is pre-placed in a coronary artery and subsequently withdrawn, and deployed rapidly if coronary occlusion occurs. The proximal stent edge sits within the aorta while the distal stent is within the coronary artery. A recent registry described 60 (0.5%) cases among 12,800 TAVI procedures and followed up outcomes over a median of 612 days. Three patients died in hospital, and two cases of stent failure were reported on longer-term follow-up.67 Longer-term complications of this technique remain uncertain.
Another option is the BASILICA technique (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVI). This involves crossing and laceration of valve leaflets by electrifying a coronary wire. It is best used to address patients at risk of coronary obstruction through direct obstruction by a leaflet or indirectly by sequestration of the aortic sinus, and can be performed in native aortic valves as well as bioprosthetic aortic valves.
Essentially, the method involves lacerating the culprit leaflet from the base to tip so that the divided leaflet lie to the sides of the coronary ostium.68 The BASILICA trial in 2019, which included 30 subjects, showed a 95% success rate in traversal and laceration, and no coronary obstruction.
A recent multicentre registry with 214 patients showed successful leaflet traversal and laceration in around 94% of patients. Procedural success was defined as successful traversal and laceration without mortality, coronary obstruction or emergency intervention, and was achieved in 86.9% of patients. Of these, 4.7% developed partial or complete coronary obstruction despite BASILICA. Of the 10 patients affected, six had partial obstruction that was relieved with stenting. In the four with complete obstruction, one patient had right coronary obstruction relieved with balloon angioplasty. Of the remaining three with left main coronary artery complete occlusion, one underwent chimney stenting and two required THV snaring and the deployment of a new valve.69
Coronary Access after TAVI
As younger patients now undergo TAVI, life expectancy after procedure has become longer. Coronary re-access has become more important in this group of patients because future acute or chronic coronary syndromes are possible.
Acute coronary syndrome rates after TAVI have been estimated to be around 10% with a follow-up duration of 25 months. All-cause mortality was up to almost 40% at 21 months’ follow-up, which may reflect patient demographics. A follow-up, multicentre study showed similar results. In these studies, coronary angiography was performed in only 60–70% of patients. This may be because of patient characteristics such as elderly age or frailty, or as previous coronary angiography showed no revascularisable lesion.70,71 Coronary angiography for chronic coronary syndromes is also likely, especially with the preponderance of CAD at baseline.
Studies so far have investigated both self-expanding and balloon-expandable valves and showed success rates of coronary angiography of over 90% for most, and high PCI success rates.72–77 Angiography of the right coronary artery may be more difficult than of the left for anatomical reasons, although success rates are still high.73,77 Small studies exploring PCI of the left main coronary artery after TAVI have also shown good success rates.55 Depending on aortic root anatomy as well as THV frame, there are different recommendations to best enable cannulation of the coronary ostia. Cannulation may be through stent cells or through entering the sinus from above the THV frame. Use of coronary wires or balloons or even guide extension catheters may be necessary.7
It is imperative to consider the patient’s long-term coronary journey when planning for a TAVI procedure. This is especially so given the likely possibility of future TAVI-in-TAVI procedures. The appropriate selection of the initial THV is of utmost concern to avoid future difficulties.
With regard to transcatheter valve choices, the self-expanding valve has an inflow that secures the frame onto the annulus, a concave central portion and a large outflow that rests in the ascending aorta. The concave central portion reduces risk of coronary occlusion by creating more space in the sinuses of valsalva.78 The height of the skirt may, however, obstruct coronary ostia acutely as well as hinder future re-access, though this may be mitigated by controlling the depth of implantation. However, valve commissure posts may also impede the ease of coronary access if they line up with coronary ostia.
Balloon-expandable valves do not have a central concavity like self-expandable valves, and have a shorter stent frame. This shorter stent frame may favour future coronary access by allowing catheters to enter the coronary sinus above the frame rather than through stent cells.77 CT-based studies have shown that balloon-expandable valve frames are infrequently positioned higher than coronary ostia. In these patients, there was no statistically significant impact on early MI rates or impact on future PCI. This has been theorised to be related to sufficient depth of sinuses such that coronary ostia are not directly opposed by the THV.79,80
Post-TAVI CT imaging may help with planning coronary access in stable patients but its use may be limited in patients with acute coronary syndromes.81,82 Such imaging studies have shown that, while bioprosthetic valve commissures are often aligned with the native commissures (and hence the coronary ostia) in surgical aortic valve replacement, TAVI neo-commissural alignment with coronary ostia is often random, with a significant concern over commissural post overlap.82–85
Commissural alignment techniques to best align neo-commissures to coronary ostia have been described. One such technique involves controlling the position of the delivery catheter before introduction through the femoral artery, as well as fine tuning while the catheter is in the descending aorta or aortic root. While some of these techniques have proven to improve alignment, current-generation devices still have significant limitations.
In the ALIGN-TAVR trial, neo-commissural overlap with one or both coronary arteries after optimisation still occurred in 24.3% of patients with the Evolut THV (Medtronic), and 12.5–14.3% with the ACURATE neo THV (Boston Scientific). Attempts at crimping the Sapien 3 THV (Edwards Lifesciences) at a fixed commissural orientation for optimisation did not show a difference in the incidence of overlap.86 Most recently, a study of CT-based optimisation of coronary alignment could suggest an advantage of aligning coronary arteries rather than commissures.87
Future Directions
There are still many gaps in the evidence in CAD management for TAVI patients. The role of revascularisation before TAVI will be better answered in future randomised controlled trials. It is likely that quantification of ischaemia will play an important role in deciding whether revascularisation is indicated, much like in CAD in general.
With regard to procedural management, THVs are constantly evolving and improving in design. Better recognition of factors that affect coronary obstruction have improved THVs in newer generations and reduced risks of procedures. It is likely that newer techniques will evolve to allow greater success rate of coronary access.
As TAVI matures, awareness is increasing that patients are likely to undergo TAVI-in-TAVI procedures in the future. With these valve-in-valve procedures, coronary obstruction risk and access difficulty will increase.88,89 Hopefully, future studies will shed light on an appropriate strategy for younger patients who have to undergo multiple such procedures.
Conclusion
CAD is common in patients undergoing TAVI. This complex interplay between CAD and TAVI involves relationships between patient risk profile, aortic root anatomy, THV design and deployment, as well as strategies for avoiding coronary complications. A deep, nuanced understanding of CAD management in TAVI patients is critical in optimising patient outcomes, in not just the immediate future but also over their expected lifetime.










