Dr Bozkurt is one of the world’s leaders in heart failure (HF). She gave a groundbreaking talk on the trajectory she would like to see the field move towards, including reconsideration of the definition of decompensated HF, a vision of how to target success for treating decompensated HF and how to create phenotypical types to tailor treatment strategy to phenotype.
Dr Bozkurt started her talk by providing the overarching definition of acute HF (AHF) based on three key steps. Step 1 requires a worsening of patient symptoms: HF is a syndrome of symptoms and signs and, by definition, AHF requires the symptoms and signs of HF to newly onset (de novo HF) or worsen (acute decompensated HF [ADHF]). Step 2 refers to location: AHF should manifest as a severe enough condition for the patient to seek urgent medical attention leading to an unplanned hospital admission or emergency department (ED) visit. Step 3 requires an escalation of care: patients with AHF require urgent evaluation with subsequent initiation or intensification of treatment, including intravenous therapies or procedures.
Dr Bozkurt noted that the definitions of AHF provided in the various guidelines do not always correspond to these three dimensions. For example, the US guidelines define AHF only on the basis of the location criteria and require the patient to be hospitalised.1 Most of the other decision pathways require all three dimensions to be met, but in differing ways. Dr Bozkurt showed how the AHF definitions relied upon for inclusion in clinical trials also differ and require different combinations of the three steps. For example, the PIONEER trial required a primary diagnosis of HF that included symptoms and signs of fluid overload.2 The GALACTIC-HF trial required patients to be currently hospitalised for HF or have made an urgent visit to the ED for a primary diagnosis of HF with elevated B-type natriuretic peptide/N-terminal pro B-type natriuretic peptide (NT-proBNP).3 In the SOLOIST trial, patients must have been admitted to hospital or had an urgent care visit to the ED, the HF unit or the infusion centre for worsening HF associated with evidence of intravascular volume overload and have received intravenous diuretics.4
These examples illustrate the variations in concepts that make the construction of clinical trials for AHF all the more challenging. In contrast, where HF is an endpoint in the adjudication of clinical trials, the definition tends to be more specific. Diagnoses are often based on standard criteria, such as the ICD-10 codes, the presence of symptoms and signs, objective diagnostic criteria such as imaging or elevated natriuretic peptide (NP) levels and escalation of treatment via intravenous diuretics, mechanical circulatory support (MCS) or other interventions. In HF clinical trials, hospitalisation has frequently been used to increase the risk profile of AHF patients. Trials have adopted a strategy to incorporate patients who have a history of HF hospitalisations to target a population with higher event rates.
Proposal 1: Change in Nomenclature
Dr Bozkurt explained how the acuity of the presentation does not necessarily matter for AHF. For example, if a de novo HF patient is not decompensated, they may not be in AHF and may not require hospitalisation; not all de novo HF is ADHF. There can be three types of patients admitted for HF: patients with de novo HF, patients with acute decompensated de novo HF and patients with decompensation of chronic HF.
Having appreciated that ‘acute’ is not a necessary requirement in decompensated HF, Dr Bozkurt explained that ‘decompensation’ is the common denominator of terminology. Decompensation is not defined by factors such as a series of hospitalisations or the haemodynamics alone, but rather the requirement for an escalation or intensification of urgent therapy, be it in a primary or secondary setting. This is the proposed change in nomenclature that Dr Bozkurt wishes to promote and gain consensus on. She proposed that decompensated HF (DHF) in the new era can be defined as HF patients who: are refractory to standard/optimised HF treatment with active deterioration; and require intensified/escalated/urgent intravenous, advanced or rescue HF therapy that is not specified by location or requirement of hospitalisation. Dr Bozkurt made the distinction between worsening HF and DHF: worsening HF responds to interventions as an outpatient and its signs and symptoms can be managed by oral HF medication titration. However, DHF involves worsening symptoms and signs that require intensified, urgent and additional therapy.5
Proposal 2: Define the Decompensated Heart Failure Haemodynamic Subset and Treat
There have been numerous conceptualisations of phenotypic classification for HF over the past two decades. Gheorghiade et al laid out the clinical presentations of AHF in eight categories.6 Nieminenan et al and McDonagh et al further defined the phenotypes of AHF.7,8 Abraham et al showed how there can be overlapping phenotypes.9 The Society for Cardiovascular Angiography and Interventions (SCAI) SHOCK stage is also relied upon as an indication of shock severity and comprises one component of mortality risk prediction and treatment decisions in AHF patients with cardiogenic shock.
Combining knowledge from these former studies, Dr Bozkurt proposed a simplified classification of haemodynamic subsets for the selection of treatment for DHF. The two haemodynamic subsets are the congested (warm and wet) and the hypoperfused (cold and wet). Once the haemodynamic subset has been defined, the patient can be appropriately treated according to their subset and urgency to achieve a positive change. The primary treatment goal for the congested haemodynamic subset should be to decongest, whereas for the hypoperfused haemodynamic subset the treatment goal should be to provide circulatory support with inotropes or MCS.
Each haemodynamic subset can each be further subcategorised; for example, the congested haemodynamic subset can be subdivided into pulmonary congestion, systemic congestion and both, with each type requiring specific therapies. The hypoperfusion haemodynamic subset can divided into pre-shock (e.g. SCAI A and B) and cardiogenic shock (e.g. SCAI C, D and E) subsets that need pharmacological or mechanical support.
Proposal 3: Define Specific Aetiology, Phenotypes and Severity
The third step that Dr Bozkurt proposed is to define the specific aetiology, phenotype and the proximate cause of decompensation that needs specific treatment in addition to HF. This requires consideration of the primary cardiac diagnoses complicated with DHF (e.g. acute coronary syndrome, pulmonary embolism, hypertensive emergency, myocarditis) and non-cardiac diagnoses (e.g. pneumonia, chronic obstructive pulmonary disease exacerbation). There is also the need to specify the ventricle type of DHF (e.g. left ventricular failure, right ventricular failure, biventricular failure) and to specify the presentation severity that needs specific therapy (e.g. DHF with respiratory failure, DHF with diuretic resistance, DHF with repeated DHF episodes). It should be noted that diuretic resistance is important and is a change to be defined as an output or during the inpatient intervention.
Proposal 4: Check the Patient Trajectory
The fourth step is tracking the trajectory of DHF patients based on their response to the initial DHF therapy. Despite the importance of patient trajectory, current administrative policies tend to prioritise discharge over improved long-term outcomes. Therefore, it is important that if patients require advanced therapies due to unresolved congestion, they are not discharged prematurely. Instead, they should only be discharged once they have achieved resolution of symptoms and signs and improvements in the diagnostic markers. The tricky part is to know what metric or markers of decongestion should be used for tracking the patient trajectory.
Reviewing the outcomes of the standard of care arm from the seven recent DHF trials, it was shown that two-thirds of patients achieve resolution of jugular vein distension (JVD) and pulmonary rales by discharge, and 50–75% have resolution of oedema by discharge. Interestingly, dyspnoea improves with standard HF treatment and weight loss is achieved with varying degrees; however, there was no apparent correlation between dyspnoea and weight loss.10–17 In addition, weight loss alone was not always associated with better long-term clinical outcomes.
The ESCAPE trial showed that pulmonary artery catheter-guided therapy did not result in survival benefit; however, it is important to note that these patients were neither shock patients nor patients with marked elevated filling pressure.18 The GUIDE-IT trial targeted NP concentrations to guide HF therapy in ambulatory patients, but was unable to demonstrate that a strategy of NT-proBNP-guided therapy was more effective than the standard care strategy in improving outcomes.19 The PRIMA II trial built upon this and demonstrated that the guidance of HF therapy to reach an NT-proBNP reduction of >30% after clinical stabilisation did not improve 6-month outcomes.20 Instead, as demonstrated in the PIONEER trial, patients who improve on guideline-directed therapy see a corresponding reduction in NP levels. In this way, NP acts as a marker for responsiveness to therapy but is not a target for therapy in itself.2 The DOSE trial demonstrated that more complete decongestion is associated with better outcomes, and patients who had improvements in two or three objective markers of decongestion had improved clinical outcomes than those with improvement in no or one marker (39.0% versus 53.8%; p=0.03).21
Dr Bozkurt’s proposal is to individualise therapy depending on the baseline and changes from baseline; one should look for improvement or resolution of symptoms, signs and diagnostic biomarkers (e.g. NP concentrations, haemodynamics, O2 saturation levels, filling pressures). Instead of using one or two metrics to track the patient response, an array of metrics should be used to accurately assess the patient’s decongestion response.
Dr Bozkurt highlighted the need to beware of false alarms (e.g. acute kidney injury, cardiorenal syndrome) that can hinder clinical care and clinical trial design. This is because creatinine can rise with successful decongestion and is not associated with worse outcomes in ADHF patients.22 However, worsening creatinine with persistent congestion, not worsening HF alone, is associated with adverse outcomes. The ESCAPE trial showed that rising creatinine accompanied by haemoconcentration is associated with better outcomes,23 whereas the EVEREST trial showed that haemoconcentration is associated with worsening HF but better clinical outcomes.24
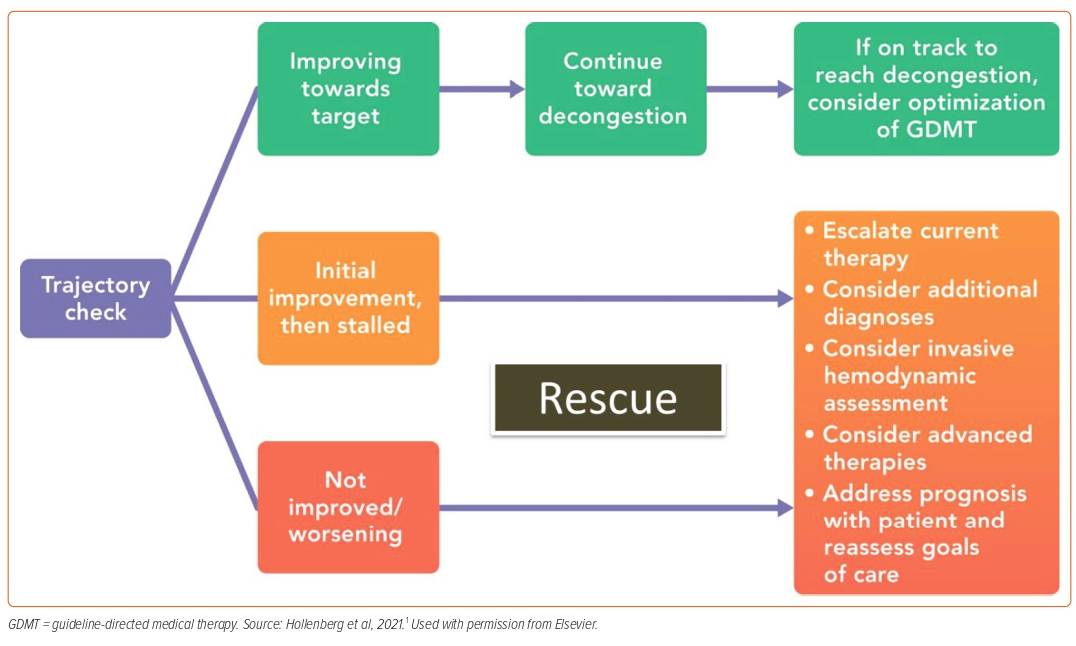
Dr Bozkurt explained one of the major problems in DHF patient management is a high rate of discontinuation of guideline-directed medical therapy (GDMT), ranging from 23% to 42% of patients following hospitalisation.25 There is a need to respond appropriately to the trajectory of the patient’s response to DHF therapy and ‘rescue’ those patients who are deteriorating. Approximately one-third of patients in the congestion subset are refractory, whereas another one-third of patients are discharged with residual congestion. Approximately 6–10% of patients in the hypoperfusion subset are refractory or worsen.26
Step 4, therefore, places an emphasis upon the patient’s trajectory over the course of hospitalisation (Figure 1).27 Refractory patients or patients who experience worsening HF after initial treatment require escalation of treatment. Dr Bozkurt defines ‘refractory’ as those patients whose condition fails to improve with initial DHF treatment or those who require escalation. Patients with worsening HF are a different population, comprising those who experience worsening HF signs or symptoms, pulmonary oedema or cardiogenic shock and those who require initiation of new, repeat or an increase in intravenous treatment, MCS or ventilatory support.
Proposal 5: Look for the Long-term Trajectories for Decompensated Heart Failure
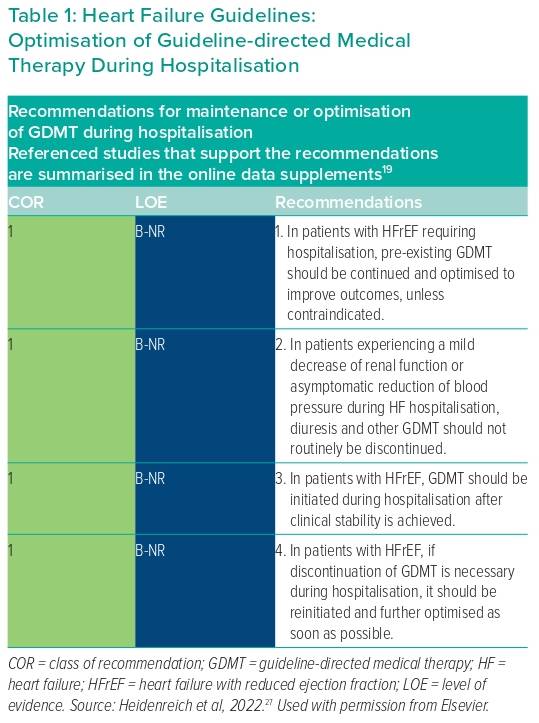
The long-term trajectory of DHF patients should be monitored and efforts should be taken to modify and change their trajectory. This step includes continuing and optimising GDMT, treating and preventing precipitating factors and preventing readmission/mortality. For patients who have had multiple admissions within the past few months (e.g. four admissions over the past 12 months or three admissions over the past 6 months), the aim is to decongest them, maintain their quality of life, optimise GDMT, prevent precipitating factors, use disease-modifying approaches and consider advanced care (e.g. transplant or MCS) or palliative care (Table 1). GDMT should always be initiated before discharge because it is safe and effective.25
In summary, Dr Bozkurt’s lecture provided a pioneering vision of reconceptualising HF. In Dr Bozkurt’s proposal, the focus should be on the ‘decompensated’ aspect of HF rather than the ‘acuity’. Decompensated HF patients are those with active deterioration despite attempts to optimise HF treatment and who require intensified or urgent intravenous, advanced or rescue HF therapy. In Dr Bozkurt’s vision, DHF is not specified by location, hospitalisation or acuity of presentation. She proposes that haemodynamic subsets of DHF patients should be defined and treated accordingly, in addition to the proximate causes, to enable therapy to be tailored to the specific cause. She reminded us of the need to recognise a confounding presentation that can hijack the ability to treat the patient appropriately and to recognise chronic and acute trajectories to ensure patients are rescued if they are refractory or worsening. In these cases, patients can be escalated to initiate a higher level of care if their life trajectory implies an active deterioration.