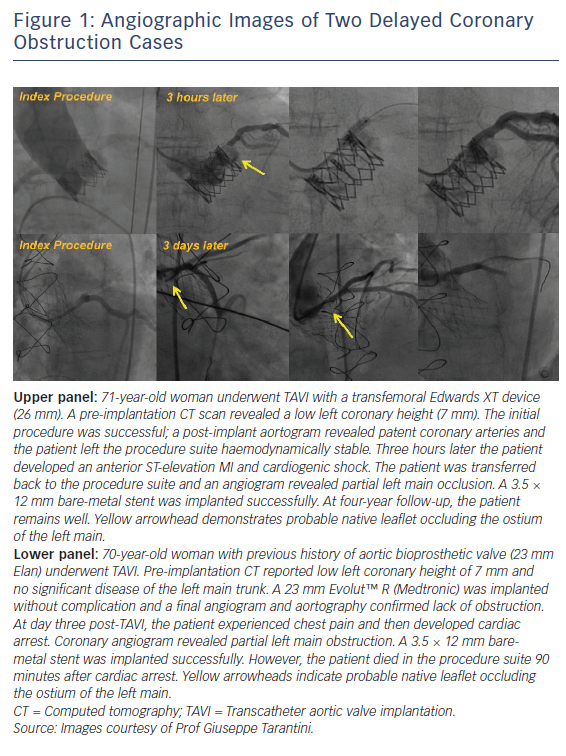
Transcatheter aortic valve implantation (TAVI) has undoubtedly revolutionised the treatment of severe aortic stenosis and has become the preferred treatment option for patients at increased surgical risk.1,2 Although outcomes have improved and complications reduced over time, certain potentially catastrophic complications remain.3,4 Coronary obstruction has long been a feared complication and is classically recognised to occur in the acute setting just after valve deployment.4 However, we recently published data from a large international registry on the incidence and outcomes of delayed coronary obstruction (DCO), a phenomenon in which the obstruction occurs in the hours, days or months following the procedure (Figure 1).5 It is important to mention that coronary obstruction is not solely related to TAVI; both acute coronary obstruction and DCO can occur with conventional surgical aortic valve replacement. The true incidence of surgical coronary obstruction is unknown but historical data have reported it to be as high as 3 %.6,7
Delayed coronary obstruction
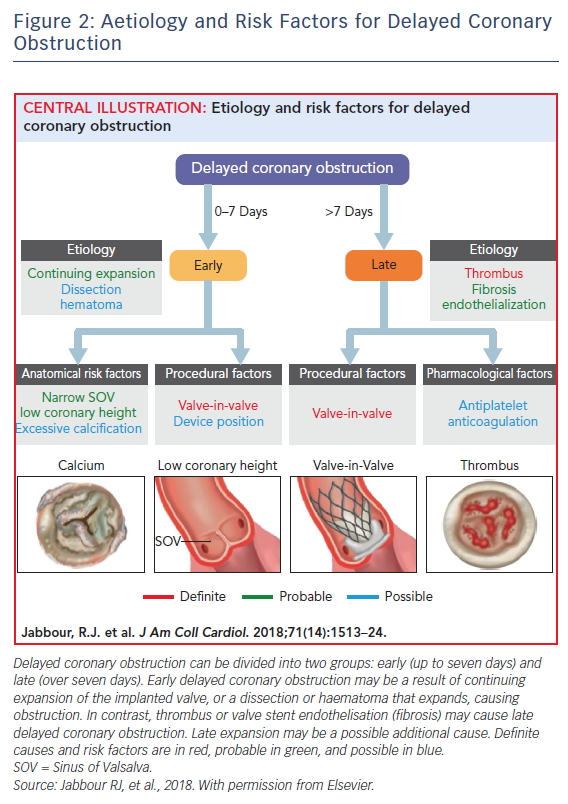
DCO is defined as: obstruction of the left main or ostial right coronary artery occurring after successful TAVI, with a diagnosis by angiogram, surgery, or autopsy to ensure adequate evidence of obstruction and, importantly, not solely related to coronary artery disease or in-stent restenosis and so directly related to the TAVI procedure or implanted prosthesis. The incidence reported from a large international multicentre registry was 0.22 % (38 cases from a total of 17,092 TAVI procedures), which is typically lower than acute coronary obstruction (up to 1 %).4,5 However, as sudden death could be the first manifestation of DCO outside of hospital and up to one-third of TAVI patients have undergone prior coronary artery bypass graft and so may be protected from native coronary obstruction, the real incidence may be higher.8 Certain similarities exist between DCO and acute coronary obstruction. For example, DCO was more frequently observed after valve-in-valve procedures (0.89 % versus 0.18 %; p<0.001) and in two out of three cases the patient had at least one known classical risk factor for acute coronary obstruction (narrow sinus of Valsalva, low coronary height or valve-in-valve procedure).5 DCO occurred more commonly if self-expanding valves were used during the index procedure rather than balloon expanding valves (0.36 % versus 0.11 %; p<0.01). DCO was most likely to occur within less than, or up to, seven days (n=24, 63.2 %; early DCO), with just over a third of cases occurring ≥60 days (n=14, 36.8 %; late DCO). Early DCO cases were likely to have unstable presentations (cardiac arrest or ST-elevation MI) while late DCO had more stable presentations (stable angina). Percutaneous coronary intervention was attempted in most cases (74.3 % left main; 60.0 % right coronary), and stent implantation was successful in 68.8 % of cases. Similarly to acute coronary obstruction, the in-hospital mortality associated with DCO was high at 50 % (n=19) and higher if DCO occurred within less than, or up to, 7 days of the index procedure (62.5 % versus 28.6 %; p=0.09).5 The absence of cases between seven and 60 days possibly indicates two distinct mechanisms. For example, early DCO may be related to the index procedure, with dislodgement of the native valve leaflets as a result of continuing expansion of the TAVI valve, dissection, haematoma or thrombus formation, while late DCO may be related to persistent inflammation, valve stent endothelisation or thrombus embolisation (Figure 2).5 The pathogenesis is broadly similar to conventional surgical aortic valve replacement, whereby acute coronary obstruction is thought to be predominantly procedurally associated (e.g. ostial cannulation to administer antegrade cardioplegia). In contrast, DCO is thought to be a result of persistent inflammation or continuous turbulent flow across the prosthesis leading to fibrous proliferation and intimal thickening in the surrounding regions.6,7 DCO has important implications for future research. As there is a drive towards use of TAVI in lower-risk patients with inevitably longer life expectancy, research is needed to characterise the phenomenon in greater detail and next generation devices should be designed to mitigate the risk.1
Implications for New Transcatheter Heart Valve Design
Direct anchoring to native valve leaflets
Most of the approved TAVI valves today use a radial force-dependent mechanism to keep the prosthesis fixed in place. However, radial force will not prevent the native aortic valve leaflets from prolapsing and causing coronary obstruction.9 Certain newer generation TAVI devices (ACURATE Neo™ [Boston Scientific], JenaValve™ [JenaValve]) are fixed in place by using a direct anchoring mechanism to either the calcified native leaflets or surgical valve leaflets, which would mitigate the risk future prolapse and coronary obstruction. In an initial early experience with the ACURATE Neo device in 30 patients with high-risk features (mean left main ostial height of only 10.8 mm and sinus of Valsalva:annulus ratio of 1.3 ± 0.8), there were no cases of coronary obstruction.10,11 Furthermore, in the Symetis ACURATE neo Valve Implantation Using Transfemoral Access (SAVI-TF) registry of 1,000 patients, no coronary obstruction events were reported at 30 days.12 Longer-term data from the ‘CE-approval cohort’, reported no coronary obstruction events in 89 transfemoral access patients at one-year follow-up.13 In contrast, clinical experience using the JenaValve is relatively limited; transfemoral device data are awaited, while initial registry data with the transapical device for the treatment of aortic regurgitation has been encouraging.14 A theoretical limitation of this mechanism is the risk of relatively increased post-procedural gradients, especially in patients with small annulus diameters. Although this has not been reported with ACURATE Neo so far, further data are awaited.9
Increased Cell Size
As there is a move towards lower-risk patients with longer life expectancies, coronary angiography or percutaneous coronary intervention after TAVI is becoming an important issue. Although this has been reported to be feasible, it can be technically challenging, especially in the case of self-expanding valves that extend above the coronary ostia.14 By designing valves with larger stent cell sizes (for example, the Portico™ valve [St. Jude Medical]), future access to the coronary circulation can be facilitated. Additionally, the persistence of turbulent flow and inflammation may cause valve stent endothelisation over time. If the cell surface area is increased there is a theoretical likelihood that the degree of coronary obstruction will be lower.
Low Profile Skirt
While valve skirts are important for minimising paravalvular leak, DCO has occurred with self-expanding valves because of prosthesis skirt obstruction.15 Theoretically, the chance of obstruction would be greater in high-implantation cases. Minimising the profile of the skirt could potentially prevent DCO.
Retrievability
Newer generation devices (Lotus™ [Boston Scientific], Portico, Evolut™ R [Medtronic]) are retrievable and are advantageous for high-risk acute coronary obstruction cases. Different degrees of retrievability exist, for example the Lotus is retrievable after full implantation, while the Portico or Evolut R can only be removed prior to complete deployment. Although retrievability is predominantly beneficial in acute coronary obstruction cases, since a prosthesis can be retrieved if there is immediate angiographic evidence of obstruction, a significant proportion of DCO patients (n=18; 47.4 %) had high implantation depths. Having a device that is fully retrievable even after deployment could be beneficial to obtain an optimal result, which may help to prevent DCO.
Patient Management
Pre-transcatheter Aortic Valve Implantation
Computed Tomography
Pre-procedural CT evaluation should now be considered almost mandatory during the TAVI evaluation process. As well as providing valuable information regarding potential vascular accesses and the aortic root, detailed information on risk factors for coronary obstruction — including sinus of Valsalva width, coronary heights, bulky calcium nodules or excessively long leaflets that may obstruct the coronary ostia — can easily be obtained.
BASILICA Technique
Recently, a novel technique called the bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) has been reported.16 This procedure is performed prior to TAVI insertion with the use of catheters to direct an electrified guidewire to traverse and lacerate through the centre of the aortic valve leaflet. The first-in-human experience was recently reported in seven patients at high risk of coronary obstruction in non-surgical candidates on a compassionate basis. Six patients had failed bioprosthetic valves, two had severe aortic stenosis, three had severe aortic regurgitation, with the remaining two having mixed aortic valve disease. Interestingly, one patient underwent laceration of both left and right coronary cusps. There was no haemodynamic compromise in any patient following the procedure and all patients had successful TAVI, with no major complications occurring and no deaths at 30-day follow-up. Further data are awaited on this promising technique.16,17
During Transcatheter Aortic Valve Implantation
Coronary protection
In a recently published DCO case series, nine cases (23.7 %) had coronary guidewire protection. Importantly, DCO can occur even if an ostial coronary stent was deployed during the index procedure; seven patients (18.4 %) had left main stent insertion during the index procedure and then developed DCO. Possible ways to mitigate this risk, which require evaluation, include excessively protruding ‘chimney’ stents or using stents with greater radial strength to prevent stent deformation from the native valve leaflets or transcatheter heart valve.5
Post-transcatheter Aortic Valve Implantation
Low Threshold For Imaging the Coronary System
As DCO can occur in the months and years post-TAVI, in patients without risk factors for acute coronary obstruction, clinicians should have a low threshold for imaging the coronary circulation. In stable presentations, coronary CT angiography should be the first-line investigation. If a patient has classical risk factors for acute coronary obstruction (valve-in-valve, low coronary heights, narrow sinus of Valsalva dimensions), increased monitoring in the peri-procedural period is recommended (for example, a CT scan before discharge).5
Anticoagulant Therapy
Thrombus embolisation from a prosthetic valve can be a cause of DCO. Also, reduced leaflet motion, which is a presumed surrogate marker of thrombus, was recently reported in 13 % of patients (n=17 from 132) from two pooled registries. So valve thrombosis is an area of intense research and interest. Even though the reduced leaflet motion resolved on follow-up CT in all patients treated with therapeutic anticoagulation, there is no recommendation for routine anticoagulation post-TAVI at present.18 The role of anticoagulation is specifically being evaluated in on-going randomised clinical trials, including the Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis (ATLANTIS) study (NCT02664649) and the Comparison of a Rivaroxaban-based Strategy With an Antiplatelet-based Strategy Following Successful TAVR for the Prevention of Leaflet Thickening and Reduced Leaflet Motion as Evaluated by Four-dimensional, Volume-rendered Computed Tomography (GALILEO-4D) trial (NCT02833948).
The rapid expansion of TAVI use over the last 15 years is likely to continue as outcomes and operator experience improve. The recent description of DCO is important, as clinician awareness has been raised. Newer valves can be designed to mitigate the risk of DCO because the occurrence of this complication, although infrequent, will be less tolerable in low surgical-risk patients with longer life expectancies. Future studies should monitor this condition to characterise it further.