The original studies of coronary balloon angioplasty performed by Andreas Gruentzig attempted to employ physiological guidance by measuring trans-stenotic gradients with over-the-wire angioplasty catheters.1 However, these catheters were of such large calibre that they contributed to the stenosis being assessed and in addition, measurements were made at rest as the importance of assessing functional stenosis severity during hyperaemia was not yet fully appreciated. The development of pressure and flow (Doppler) sensing coronary guide wires in the early 1990s led to a re-emergence of physiologically guided percutaneous coronary intervention (PCI). A series of studies using Doppler-derived measurements of coronary flow velocity to calculate coronary flow reserve (CFR) were conducted with variable results, partly related to the technical difficulty of recording stable flow velocity signals but mainly to the fact that CFR cannot separate out the contribution to inadequate myocardial blood flow of resistance in the stenotic large-calibre epicardial conduit vessels (the target for intervention) from that in the microcirculation. The concept of pressure-derived fractional flow reserve (FFR) was first described by Pijls et al. in 19932 and following proof-of-concept studies, several clinical trials demonstrated that the use of FFR to guide PCI was not only associated with improved clinical outcomes but was also highly cost effective.3–6 Despite this, the worldwide use of FFR remains disappointingly low at around 5–10 % of all PCIs.7 This figure has been confirmed in a large recent survey by Toth et al.8
Various reasons have been proposed for the low utilisation of FFR, including the cost of the pressure wires as well as the cost, inconvenience and side effects associated with the induction of hyperaemia. In response to this, investigators have explored the ability of resting pressure-derived indices of stenosis severity to predict FFR and thereby to potentially remove the need for hyperaemia, at least in some patients. In this review we will discuss the role of one of these indices, the instantaneous wave-free ratio (iFR), and examine the strengths and weaknesses of iFR and FFR in detail with emphasis on the available evidence to date.
Physiology
FFR is the ratio of mean distal coronary pressure and mean aortic pressure measured during maximal hyperaemia that is generally achieved through administration of a potent vasodilator such as adenosine, ATP or papaverine either by IV infusion or by intracoronary (IC) bolus injection. By rendering myocardial microvascular resistance constant and minimal, it is possible to separate out the impact of disease in the epicardial conduit artery on myocardial blood flow2.
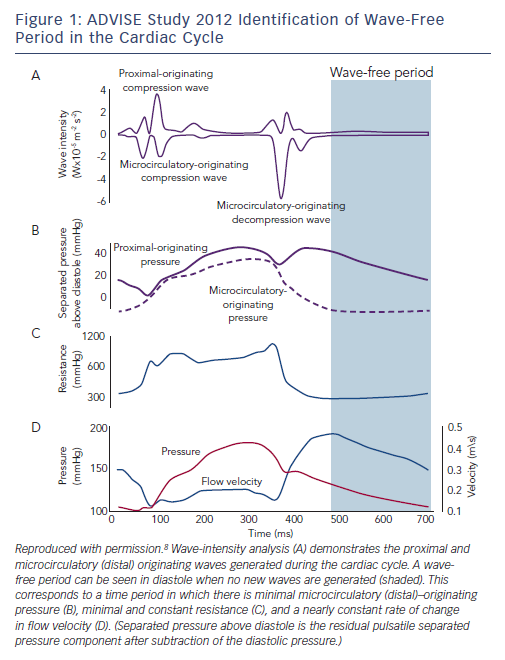
iFR was first described in 2011 following a proof-of-concept study in 157 coronary stenoses in which FFR was compared with resting (non-hyperaemic) trans-stenotic pressure gradients measured in a time period beginning 25 % into diastole and ending 5 ms before the onset of systole, the ‘wave-free’ period.9 It was proposed that coronary microcirculatory resistance was already constant in this window and as low as whole-cycle resistance during hyperaemia (Figure 1). In theory, this should allow accurate prediction of FFR, and in this initial study iFR was found to have a strong correlation with FFR with a receiver operating
characteristic area under the curve (ROC AUC) of 93 % for iFR≤0.83 predicting FFR≤0.80.9 For decision making in the cath-lab, the investigators proposed that an iFR value of 0.83 could be equated to an FFR value of 0.80.
Evidence to Support the Use of FFR
The original validation of FFR demonstrated that below a cut-off value of 0.75 there was 93 % concordance between FFR and a triple gold standard of exercise stress ECG, single-photon emission computed tomography and dobutamine stress echo performed before and after PCI.3 The DEFER study clearly demonstrated that it was safe to postpone intervention on lesions with an FFR≥0.75.5 The FAME study demonstrated that in patients presenting with multi-vessel PCI, FFR guidance was associated with reduced rates of mortality or myocardial infarction (MI) at 2 years: 12.9 % in the angiography-guided group versus 8.4 % in the FFR-guided group
(p=0.02).4 The FAME 2 study demonstrated a markedly reduced rate of death, MI, and urgent revascularisation in patients with stable angina with at least one lesion and an FFR≤0.80 when treated by PCI plus optimal medical therapy compared with optimal medical therapy alone: 4.3 % versus 12.7 % (HR 0.32; 95 % CI 0.19–0.53; p<0.001). In a subsequent landmark analysis of the clinical outcomes from day 7 to 2 years, essentially excluding periprocedural type 4 MI, the combined endpoint of death and non-fatal MI was significantly reduced by PCI (4.6 % versus 8.0 %; p=0.04) despite a 40 % crossover from medical therapy alone to PCI.5
FFR Beyond Physiological Assessment
FFR assessment provides more than just physiological information. The SYNTAX score is a widely used tool that can stratify patients into low, medium and high risk for PCI as opposed to surgical revascularisation.10 The functional SYNTAX score combines FFR data with
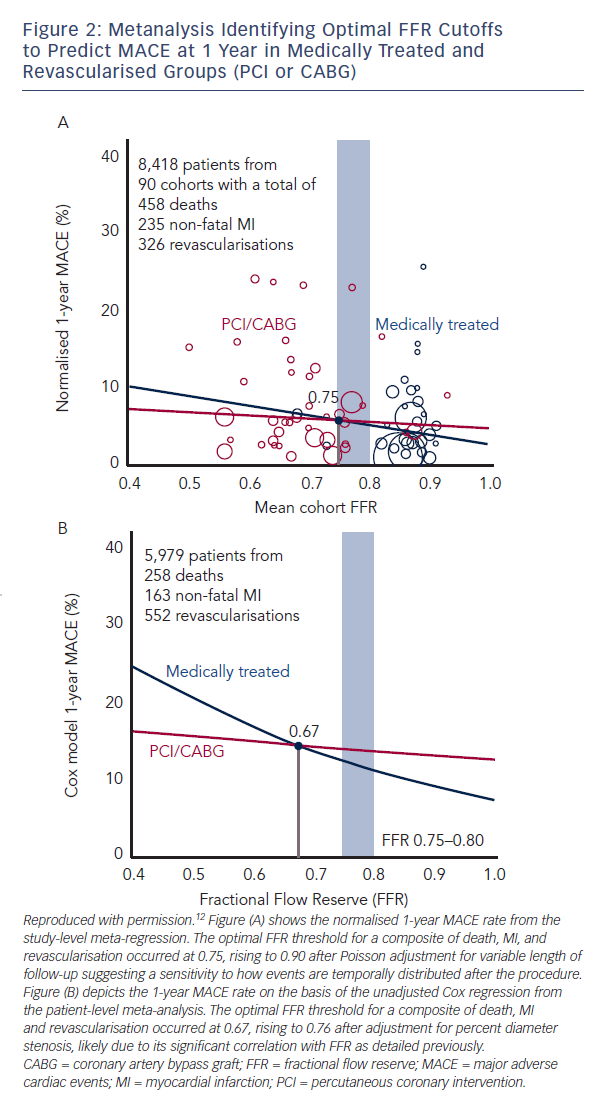
the angiographically derived SYNTAX score and potentially facilitates the selection of patients who will have equivalent outcomes with PCI or surgery.11 In terms of predicting an overall prognostic benefit from PCI, a recent meta-analysis with a combined endpoint of death, MI and revascularisation demonstrated that FFR<0.75–0.76 was associated with a clear beneficial effect of elective revascularisation (Figure 2).12
FFR at the Time of Diagnostic Angiography?
The data from the numerous trials mentioned above have firmly established the safety of FFR guidance in patients already referred for PCI. This has been supplemented by a large French registry in over 1,000 patients who had at least one intermediate lesion of 35 %–65 % diameter stenosis with overall treatment strategy recorded before and after FFR in addition to follow-up for 1-year endpoints.13 Final management changed in 43 % of cases including 33 % of patients initially assigned to receive no revascularisation, 56 % of patients assigned to PCI and in 51 % of patients assigned to coronary artery bypass graft surgery (Figure 3). Major adverse cardiac event rates (death, MI or unplanned PCI) were similar at 1 year for patients whose treatment was reclassified and those
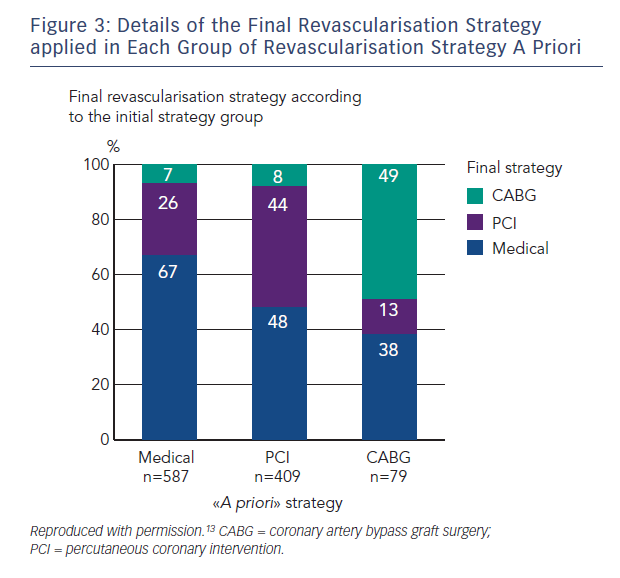
who remained in their original treatment group following FFR (11.2 % versus 11.9 %; p=not significant).13 Similar findings were reported in the RIPCORD study from the UK.14
Problems with FFR
Inducing Hyperaemia
The debate regarding the clinical challenges with adenosine continues and is mainly focused on patient discomfort, cost and additional procedural time. The reality is that most patients, with the exception of those with severe asthma, tolerate adenosine15 and use of intracoronary adenosine in particular is associated with little additional procedural time or cost while preserving diagnostic accuracy. Other agents including regadenoson and nicorandil are also options for inducing hyperaemia and in some situations may be preferable to adenosine.16 There are theoretical concerns that some of the agents used to induce hyperaemia
may introduce haemodynamic disturbances including hypotension and bradycardia, which may alter FFR and lesion classification. This was not borne out in a recent study which demonstrated no significant differences in FFR between a variety of hyperaemic agents and routes of administration.16 A strong correlation was found between IV adenosine/ATP and IC nicorandil (r=0.962; p< 0.001 with a classification agreement of 91.2 %). Similarly, IV adenosine and IV regadenoson compared well (r=0.990; p<0.001 with a classification agreeement of 100 %).16
Cost
The technology itself remains reasonably expensive; however, it is likely to become significantly cheaper with increased competition as new wires from alternative manufacturers come to the market. In addition, a pressure-sensing microcatheter is now commercially available. It is important to note that FFR guidance reduces inappropriate PCI and increases quality of life and is therefore cost effective in patients scheduled for multi-vessel PCI despite the associated expense of the pressure wire and hyperaemic agents.17
Grey-Zone FFR Values
The original validation study as well as many subsequent reports confirmed a very high concordance for non-invasive evidence of ischaemia with FFR values ≤0.75.3,5 However, in order to improve sensitivity and minimise the risk of under-treatment an FFR cut-off value of ≤0.80 was adopted in the FAME and FAME 2 trials.3,4 This has resulted in the de facto creation of a so-called FFR grey-zone between 0.75 and 0.80 within which the need to perform revascularisation is unclear and there may in fact be over-treatment. Indeed, preliminary data from our group suggests that only one-third of patients with grey-zone FFRs have perfusion abnormalities in the corresponding artery when assessed using stress MRI.18 All 33 grey-zone stenoses in a recent study were found to be non-ischaemic using a combined reference standard of hyperaemic stenosis resistance index and myocardial perfusion scintigraphy.19 Another recent study assessed 3-year clinical outcomes in 150 patients with intermediate coronary lesions. Of these patients 56 had FFR 0.75–0.80 while the remaining 94 patients had FFR >0.80.20 Although target vessel revascularisation was higher in the patients with FFR 0.75–0.80 (14 % versus 3 %; p=0.020), there was no difference in rates if death or MI. This finding is consistent with the relationship between FFR and prognosis outlined above12 and emphasises that although there is a need for subsequent revascularisation in this group, they require careful consideration of the risk:benefit ratio of intervention.
Studies Comparing iFR with FFR
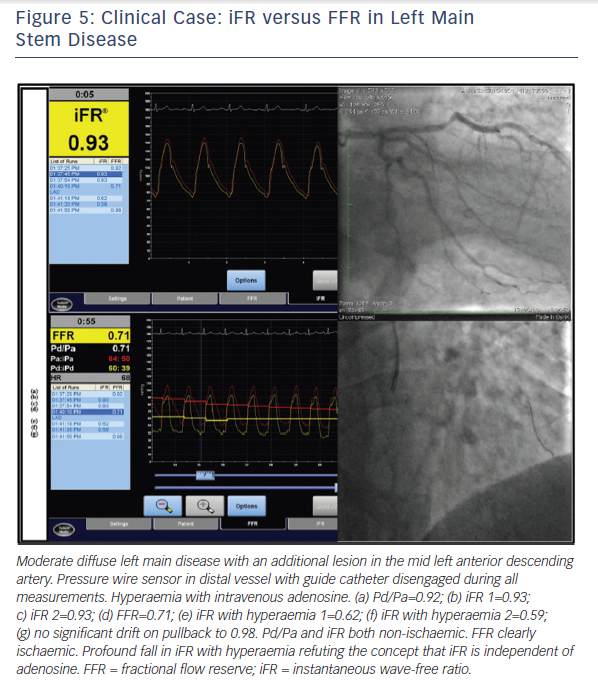
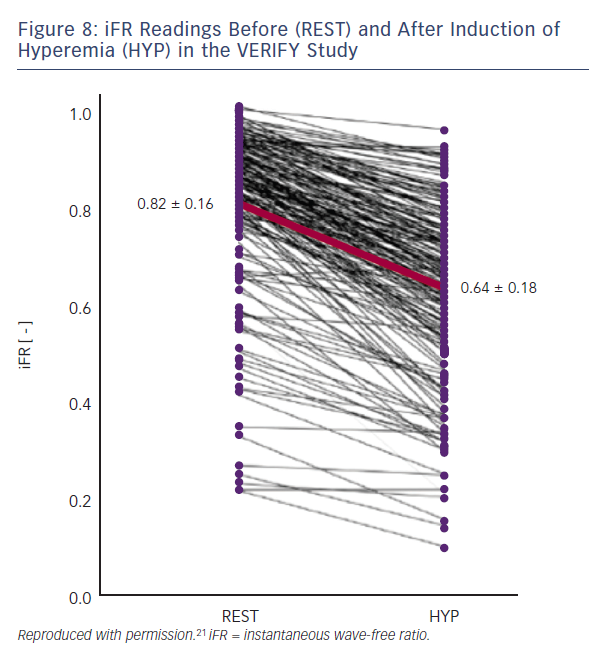
The VERIFY study demonstrated that, in a consecutive cohort of 206 coronary stenoses of intermediate severity and compared with the FAME trial FFR cut-off value of ≤0.80, the diagnostic accuracy of iFR ≤0.80 was only 60 %.21 The ADVISE clinical registry reported that in 339 stenoses a different iFR cut-off of 0.89 provided a classification agreement between iFR and FFR of 80 % for the same FFR cut-off value of ≤0.80.22 The ADVISE 2 study compared iFR and FFR in 690 coronary stenoses.23 This dataset was used to derive a proposed hybrid strategy, which correctly classified 94 % of lesions by accepting an iFR>0.93 as being negative (non-ischaemic) and an iFR<0.86 as positive (ischaemic) and only requiring FFR to be measured in lesions with iFR values in between these limits. Compared with an FFR for all strategy, this avoided the use of adenosine in 70 % of stenoses (65 % of patients) at the expense of making a different treatment decision in 1 in 16 lesions.23 The RESOLVE study was an independent core laboratory re-analysis of coronary pressure recordings from 1768 patients drawn from several studies including both ADVISE and VERIFY.24 In the RESOLVE study, the diagnostic accuracy of iFR was 80.4 % at an optimal iFR binary cut-off value of ≤0.90. It was also demonstrated that to achieve ≥90 % diagnostic accuracy at each extreme, the adenosine-free iFR range had to be restricted to ≤0.88 and ≥0.93, which was only applicable to 65 % of the study lesions.24 (Figure 5)
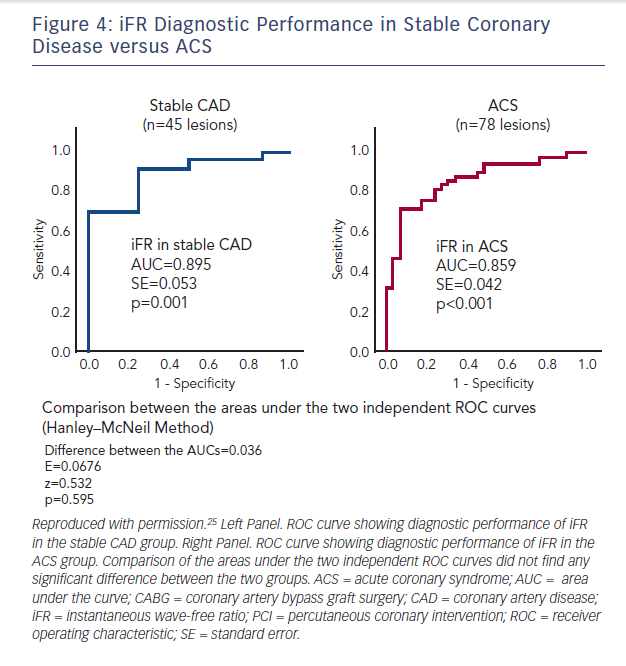
In a prospective study of 53 patients with an acute coronary syndrome (ACS) and 29 patients with stable angina, FFR and iFR in the non-culprit lesions of the ACS patients were calculated by two blinded operators.25 Using binary cut-off values, they demonstrated 81.3 % diagnostic concordance with FFR with positive and negative predictive values for iFR of 67 % and 89 %, respectively. Diagnostic accuracy was similar in both stable and unstable patient groups but was greatly improved by adoption of a hybrid approach with adenosine administration when iFR was 0.86–0.94, inclusive (Figure 4).25
It has previously been stated that the reason the VERIFY study reported an overall diagnostic accuracy for iFR versus FFR of only 60 % compared with 88 % in the ADVISE study is because in VERIFY, iFR was calculated using an in-house algorithm written in MatLab rather than the commercially available proprietary software.21 In fact, all raw data from VERIFY were provided to the independent core laboratory in the University of Columbia, New York for the RESOLVE study analysis. The correlation between iFR and FFR was stronger in the VERIFY study (r2=0.70 using the MatLab programme) than in the RESOLVE study (r2=0.66 using the proprietary software).24 Furthermore, the RESOLVE core lab analysis of the VERIFY dataset showed a mean difference in iFR values between the two different methodologies of -0.007, which is less than the mean difference between repeated measures of either parameter and therefore represents no difference at all.
iFR versus Perfusion Scintigraphy and/or Hyperaemic Stenosis Resistance as the Reference Standard
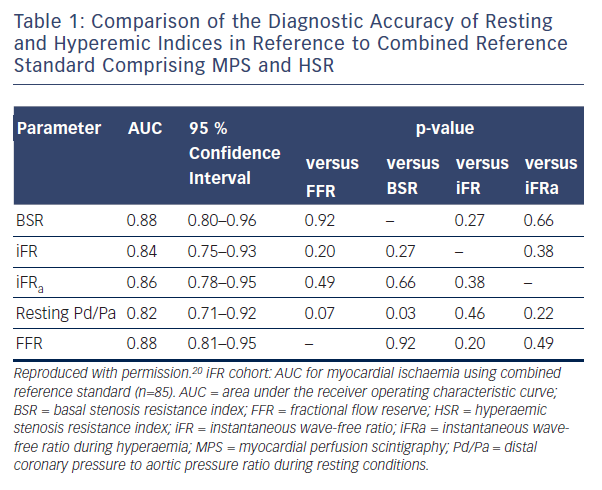
A retrospective analysis of 85 stenoses with adequate resting measurements from which to derive iFR out of a dataset of 299 combined pressure and doppler flow coronary physiology studies was performed and compared with a combined reference standard comprising myocardial perfusion scintigraphy (MPS) and hyperaemic stenosis resistance index (HSR).19 Both of HSR and MPS needed to be positive for the lesion to be considered ischaemic. The optimal ischaemic cut-off values were 0.66 mmHg/cm/sec for basal stenosis resistance (BSR) (sensitivity 84.6 %, specificity 79.7 %), 0.82 for iFR (sensitivity 69.2 %, specificity 88.1%), and 0.75 for FFR (sensitivity 88.5 %, specificity 76.3 %). There was no significant difference in AUC for either resting parameter (BSR or iFR) and FFR with respect to the combined reference standard (Table 1).19
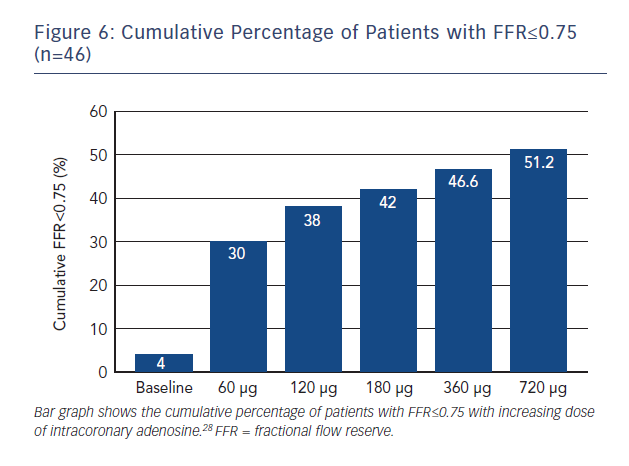
Although HSR has previously identified as being highly sensitive and specific for ischemia,26 this particular study has a number of significant limitations including a small sample size and the fact that the original validation of HSR was itself based on the MPS. This imaging modality has a high false-positive rate and importantly it was not repeated post revascularisation, which is possiby the most favourable means of assessing the diagnostic accuracy of any test for ischaemia. In addition, intracoronary adenosine was used to measure FFR in the low dose range of 20–40 μg. Previous studies have demonstrated that intracoronary doses of at least 100–200 μg are required to approximate the FFR achieved by IV adenosine infusion (Figure 6).27 This may lead to an underestimation of FFR, which in turn will erroneously increase the levels of agreement between the hyperaemic and non-hyperaemic indices. Sub-maximal hyperamiea would also be consistent with the absence of any difference between iFR and iFR during hyperaemia, a result entirely inconsistent with previously published data using IV adenosine. This study, however, showed no difference between Pd/Pa and iFR, consistent with the results of the RESOLVE meta-analysis.24
The CLARIFY registry reported data from 51 stenoses that had also been interrogated using combined pressure and flow velocity recordings.28 The authors defined HSR as the reference standard for ischaemia and determined that iFR at a cut-off value of <0.86 had an ROC AUC of 0.93 (95 % CI 0.85–1.00), while FFR<0.75 had an ROC AUC of 0.96 (95 % CI 0.89–1.00).29
iFR Versus Coronary Flow Velocity Reserve as the Reference Standard
In the JUSTIFY study, a retrospective dataset compared iFR with FFR in terms of their ability to predict coronary flow velocity reserve (CFVR).30 While CFVR is a useful parameter, it has generally been superseded by FFR due to marked dependence on systemic haemodynamics (heart rate, blood pressure, contractility) and difficulties in obtaining reproducible flow measurements. It is known that at least 30 % of lesions have discordant CFVR and FFR results and therefore it was unsurprising that FFR ROC AUC was 0.72 (CI 0.65–0.79). In this population iFR appeared to have a stronger correlation with CFVR with an ROC AUC of 0.82 (CI 0.76–0.88; p<0.001).30 The scenario of a lesion with a conventionally ischaemic FFR value but ‘preserved’ CFVR will be discussed later in this review.
Innovations with iFR
A particularly challenging group to assess physiologically are patients with tandem lesions or diffuse disease. In a proof-of-concept study coronary arteries were assessed using a motorised pullback system with calculation of iFR values per millimeter length of vessel.31 The investigators suggested that iFR may have the potential to be used in PCI planning for the determination of physiological lesion length as well as predicting the physiological response to PCI of single lesions when a number of tandem lesions are assessed.31 Given that iFR can be performed rapidly in multiple vessels, there are also potential practical and theoretical advantages in bifurcation disease.
Resting Pd/Pa
The relationship between resting gradient (Pd/Pa) and FFR was described in advance of the development of iFR in a retrospective analysis of 528 pressure-wire studies in 483 patients demonstrating a strong correlation between resting Pd/Pa and FFR (Spearman’s rank correlation coefficient 0.74).32 In fact, it was shown that a resting Pd/Pa of ≤0.87 had a positive predictive value (PPV) of 100 %, while a resting Pd/Pa of ≥0.96 had a negative predictive value (NPV) of 93.9 % in reference to FFR≤0.80. Similarly, when FFR ≤0.75 was used to define an ischaemic stenosis, Pd/Pa≤0.85 had a PPV of 95 %, while a resting Pd/Pa of ≥0.93 had an NPV of 95.7 %. The authors calculated that using these cut-off values, adenosine interrogation would have been avoided in 53 % of the FAME study population (FFR≤0.80) and 34 % of the DEFER study population (FFR<0.75), effectively coining the hybrid method for pressure wire assessment.32 Another, more recent, study retrospectively analysed 570 lesions in 527 patients applying a Pd/Pa adenosine zone range of 0.87–0.99 to achieve 100 % diagnostic accuracy with reference to FFR≤0.80, which would have avoided the use of adenosine in only 14.6 % of lesions.33 The authors reported that with a Pd/Pa adenosine zone of 0.88–0.96, 46.8 % of lesions would have been assessed without adenosine while maintaining 96 % agreement with an FFR for all strategy.
Resting Pd/Pa Versus iFR
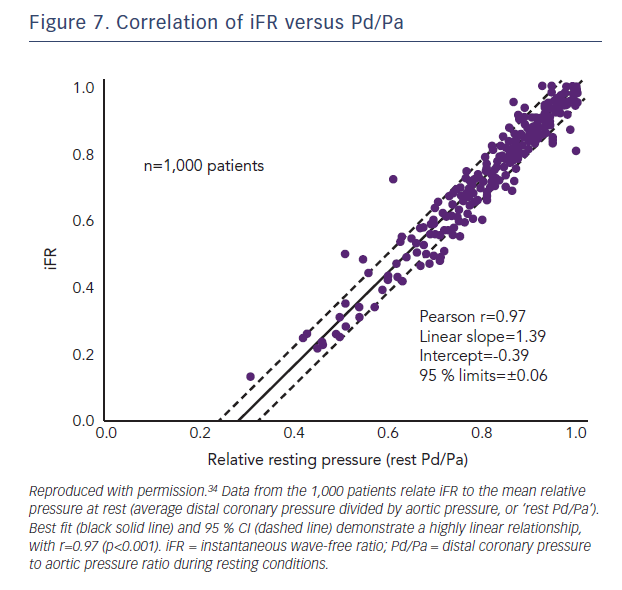
In the large RESOLVE meta-analysis, which used an independent core-lab, no significant difference was found between iFR and Pd/Pa in reference to FFR≤0.80 with an overall diagnostic accuracy of 80 % for both indices (using the optimal ROC determined cut-off points of 0.90 for iFR and 0.92 for Pd/Pa.24 The relationship between iFR and Pd/Pa was explored again in a separate study of 1,000 patients with no observable diagnostic advantage of using iFR over Pd/Pa (Figure 7).34 These are important observations for operators who elect to trade off concordance with FFR against the need for hyperaemia, as although iFR analysis is currently only possible using proprietary software, all pressure wire systems are capable of providing resting Pd/Pa.
Problems with iFR
iFR is not Independent of Hyperaemia
iFR assumes that microvascular resistance is already constant and minimal in the wave-free period. Both the VERIFY study and the analysis from Johnson et al. clearly demonstrate that this is not the case.22,34 In the VERIFY study, average iFR values fell significantly following the induction of hyperemia from 0.82 ± 0.16 versus 0.64 ± 0.18 with hyperaemia (95 % CI 0.17–0.20; p=0.0001). At an individual level, iFR decreased with hyperaemia in 97 % of the lesions studied (Figure 8). In the study by Johnson et al, myocardial resistance during the diastolic wave-free period, calculated from simultaneous pressure and flow data, was 250 % higher at rest than during hyperaemia.34 The authors recommend that if clinicians have access to iFR in their cath-labs they should measure at rest and again during hyperaemia; this will make it abundantly clear that it is not, as advertised, an ‘adenosine-independent index of stenosis severity’.
Loss of Diagnostic Accuracy
When binary cut-off values are used for decision making, as in the ongoing randomised trials of iFR guidance versus FFR guidance, DEFINE FLAIR (NCT02053038) and iFR Swedeheart (NCT02166736), the currently available data clearly confirm that avoiding the use of adenosine altogether translates into a different treatment decision in 20 % of lesions compared with an FFR for all strategy. Given wide enough non-inferiority margins and the knowledge that 80 %
of the population recruited will have convergent iFR and FFR results, it is likely that these studies will show non-inferiority. However,
such a result would conceal a mix of both inappropriate PCI and incomplete revascularisation in the iFR-guided group compared with an FFR-guided strategy.
To the authors’ knowledge, there are no randomised controlled trials comparing the hybrid iFR/FFR decision tree with an FFR for all strategy. SYNTAX II (NCT02015832) does employ this option but is an observational study. The currently available data are less comprehensive than for binary cut-off decision-making but avoiding the use of adenosine in between 50–60% of patients may translate into a different treatment decision in around 10 % of patients. Again, this will conceal a mixture of inappropriate PCI and incomplete revascularisation.
Beyond these concerns, if iFR was employed at the time of initial diagnostic angiography, as in the RIPCORD study, then patients will likely have divergent management decisions compared with an FFR for all strategy.14 This will translate not just into different patterns of stent implantation but different overall management in terms of which patients undergo revascularisation and by which technique.
Whether employed in patients already scheduled for PCI or within a binary cut-off or hybrid strategy there is another major concern about using iFR or indeed any resting index of stenosis severity to determine the need for revascularisation. The flow increase associated with hyperaemia when interrogating a large-calibre vessel with a proximal stenosis is much greater than that of a small diameter vessel supplying a small myocardial territory.35 This means that some prognostically important lesions may exhibit small resting pressure gradients and therefore non-ischaemic iFR results which become manifestly ischaemic FFR results during hyperaemia. On the basis that some of these lesions have ‘non-ischaemic’ CFR values >2.0, it has been argued that the FFR result is false and the iFR result is correct. Thus, in the study by van de Hoef et al, half of the patients with discordant iFR and FFR values were ‘ischaemic’ according to the combined reference standard of MPS/HSR, and the other half were ‘non-ischaemic’.20 Notwithstanding the limitations of this study as described above, this indicates that approximately 50 % of treatment decisions will likely be incorrect. Furthermore, a CFR >2.0 does not in any way exclude myocardial ischaemia and may still be too low for a given coronary vessel and myocardial territory at high myocardial oxygen demand.
Recommendations
iFR was originally described as an adenosine-independent index of stenosis severity, but it has since been unequivocally demonstrated otherwise. The use of iFR is promoted by some groups on the grounds that the loss of diagnostic accuracy is an acceptable price to pay in return for reducing (hybrid iFR/FFR) or avoiding altogether (binary iFR) the need to use adenosine. Given the large evidence base for FFR that establishes clinical efficacy, cost-effectiveness and prognostic benefit, we do not consider this to be an acceptable trade-off. Beyond this, there are currently no clinical outcome studies with iFR. As such, it is counterintuitive and contrary to current clinical guidelines to recommend iFR over FFR. IV adenosine is the gold standard technique for inducing steady state maximal hyperaemia, but if operators do not wish to use it due to concerns over cost, time or side-effects, we suggest a policy of high-dose IC adenosine, which should minimise procedural delays as well as costs while providing high-quality data with minimal adverse effects. Healthcare providers need to examine their reimbursement policies to enable cardiologists to utilise this proven adjunct to PCI to the benefit of patients without financially stressing their institutions. n