Proceedings of Two Satellite Symposia Held at EuroPCR in May 2015 in Paris
The use of second-generation polymeric metallic drug-eluting stents (DES) in percutaneous coronary intervention (PCI) is now routine practice and has demonstrated excellent safety and efficacy compared with first-generation DES. These stents have enhanced PCI procedures, enabling the treatment of more complex lesions and clinical cases. However, specific patient subsets still require procedural and/or technological refinement to further enhance success rates and longterm patient-centred clinical outcomes. Two symposia at EuroPCR in May 2015 in Paris focused on how polymer-free DES may increase safety in PCI requiring short duration of dual antiplatelet therapy (DAPT) and are more efficacious in the setting of patients with diabetes mellitus (DM). Patients with diabetes are particularly challenging in terms of PCI, as they tend to have worse outcomes compared with patients without diabetes.
In order to meet these clinical challenges, a novel DES was discussed: the Cre8TM (manufactured by CID Spa, member of Alvimedica Group), which features controlled polymer-free amphilimus formula elution from abluminal reservoirs on the surface of the stent. These unique and innovative features may enhance clinical outcomes in patient subsets, such as those with DM, and may represent a step forwards in treating these patients.
The Latest Available Data on Polymer-Free Drug-eluting Stent Technology in Patients with Diabetes
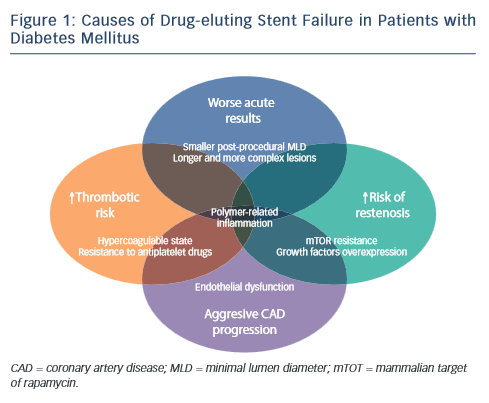
Gennaro Sardella of Rome, Italy, introduced his presentation by outlining the causes of DES failure in patients with DM (see Figure 1). These include a prothrombotic state and resistance to antiplatelet drugs, for which prasugrel and ticagrelor may be indicated. Other causes include diffuse coronary artery disease (CAD), negative remodelling and smaller minimal lumen area (MLA) post procedure, as well as extensive CAD with more complex lesions and CAD progression in the non-stented segment. The latter may be managed by the use of a coronary artery bypass graft (CABG) and statins. Persistent polymer-related arterial inflammation may be overcome by the use of a polymer-free or absorbable polymer DES. However, these patients typically present with neointimal hyperplasia and impaired rapamycinanalogue inhibition, suggesting the need for a more efficacious DES – ideally a DES designed for patients with DM.
The clinical efficacy of the Cre8™ DES was evaluated in the International Randomized Comparison Between DES Limus Carbostent and Taxus Drug-Eluting Stents in the Treatment of De Novo Coronary Lesions (NEXT) clinical study, with a primary endpoint of 6-month angiographic in-stent late lumen loss (LLL). The study achieved its primary endpoint of non-inferiority compared with a permanent polymer DES Taxus (Liberté, Boston Scientific, US). However, the most striking finding of this study was that the LLL in the subgroup with diabetes was comparable to that in the general study population, a finding that had not been seen with other DES.1 At 3 years in the clinical follow-up a 37 % decrease in target lesion revascularisation (TLR) was reported in the Cre8™ group versus the Taxus group and a reduction of 68 % was seen in the population with diabetes. These findings led to further investigation of the Cre8™ DES in the population with diabetes.
Another clinical study, ranDomizEd coMparisOn betweeN novel Cre8™ DES and BMS to assess neoinTimal coveRAge by OCT Evaluation (DEMONSTR8), assessed the Ratio of Uncovered to Total Stent Struts Per Cross Section (RUTTS) score, determined by optical coherence tomography (OCT) at 1 and 3 months for Cre8™ and a bare metal stent (BMS), and found that the Cre8™ DES at 3 months has comparable strut coverage to the BMS at 1 month, while preserving a greater efficacy in neointimal formation reduction.2
A propensity matched study (San Raffaele Scientific Institute, Milan, Italy) compared clinical outcomes of 187 real-world patients treated with the amphilimus polymer-free Cre8™ stent versus 150 patients receiving new-generation everolimus-eluting stents (EES) during the same period. It found superior clinical outcomes with Cre8™ compared with an EES in patients with diabetes.3
The MultIceNtric and RetrospectiVe REgiStry in ‘real world’ paTients with polymer-free drug elutInG stent Cre8™ (INVESTIG8) study has as its primary endpoint the incidence of clinical composite endpoint from baseline procedure to 12 months (cardiac death/ target vessel MI/clinically driven TLR). Secondary endpoints include the incidence of a clinical composite endpoint from the baseline procedure to 12 months (all deaths/all MI/any revascularisation); and the incidence of stent thrombosis from baseline procedure to 12 months, classified according to the Academic Research Consortium (ARC) definition. A recent subanalysis indicates that at 1 year, the composite endpoint was seen in 3.5 % of the population without diabetes and 5 % of those with diabetes. Clinically driven TLR was reported in 1.6 % of the population without diabetes versus 1.4 % of the population with diabetes. Freedom from events was seen in 97.8 % of the population without diabetes study and 95.6 % of those with diabetes. Definite and probable stent thrombosis occurred in only 0.6 % of the population without diabetes and 1.4 % of the population with diabetes.
In the Randomized Trial Comparing Reservoir-Based Polymer-Free Amphilimus‐Eluting Stents (AES) versus Everolimus-eluting Stents with Durable Polymer in Patients with Diabetes Mellitus (RESERVOIR) study, 112 patients with diabetes receiving glucose-lowering agents, were randomised to an amphilimus-eluting stent (AES) (Cre8™) stent or an EES with non-erodible polymer. The primary endpoint was neointimal volume obstruction at 9 months, evaluated by OCT. Secondary endpoints included strut coverage, angiographic in-stent late lumen loss and clinical endpoints such as target vessel revascularisation and probably/definite stent thrombosis.4,5 Results show that the Cre8™ was non-inferior to the EES and demonstrated non-significant superiority in the primary endpoint. In addition, the Cre8™ resulted in a lower LLL with a lower standard deviation, though without achieving statistical significance.
In conclusion, the ideal DES should combine safety with an increased DES efficacy. The Cre8™ DES has unique safety features (polymerfree structure, abluminal reservoir technology) and unique efficacy features (polymer-free structure and the amphilimus formulation), and is currently being tested in a large clinical programme. Data obtained so far suggest that the best application of Cre8™ seems to be the subset of patients with diabetes.
Live Case of a Patient with Diabetes Mellitus
This was a case of multivessel disease in a patient with DM. The patient had a prior history of atrial fibrillation (AF) and was taking oral anticoagulants. He was also suffering from hypertension, hyperlipidaemia and angina. An electrocardiogram (ECG) showed AF with ventricular response. Blood tests showed high glucose and glycated haemoglobin (HbA1c) levels and slightly abnormal cholesterol levels. The patient presented with angina (Canadian Cardiovascular Society [CCS] class 2). Angiography showed calcified stenosis of the proximal left anterior descending (LAD) artery and a double bifurcation stenosis of the marginal artery.
The primary aim of the intervention was to treat the long lesion of the LAD. The lesion was wired using regular wire then dilated using a 2.0 balloon. A long stent was used (Cre8™ 46 mm), which crossed the lesion easily. There were two bifurcation lesions: one proximal LCX and one distal. The marginal branch lesion was dilated with a 2.5 balloon and a single stent was used with good result. The aim was to put a stent on the main branch and the use of two stents was considered an alternative possible solution. A 2.5 mm x 20 mm Cre8™ stent was used for the proximal lesion; a 2.75 mm x 8 mm stent was used for the distal lesion. The artery was successfully opened. A 3.25 mm stent was used for the distal circumflex lesion. A radial approach was selected because the patient was in AF taking oral anticoagulation.
The use of the Cre8™ stent should allow cessation of DAPT at 3 months and to continue with a single antiplatelet agent as well as oral anticoagulation. Closure of the left atrial appendage (LAA) was discussed as an option if an issue arises with triple therapy.
Polymer-free Technology in Patients with Diabetes Mellitus – Procedure and Clinical Outcome
Rafael Romaguera of Barcelona, Spain, presented the case of a 62-yearold man with angina and DM, for which he was receiving insulin therapy. The patient was admitted with acute coronary syndrome (ACS) and 50–60 % stenosis in the LAD. The physician advised the patient that if a DES was used, he would do better than with BMS; that a secondgeneration DES is better than a first; that an EES has the most robust evidence of safety; and that he would be prescribed new antiplatelet drugs and statins. However, despite these interventions, he would remain at high risk of restenosis and adverse cardiovascular events. A 3.0 mm x 15 mm second-generation DES (zotarolimus-eluting stent [ZES]) was implanted. At 6-month follow up, the patient had stable angina. A 3.5 mm x 15 mm paclitaxel-eluting balloon was utilised to treat the restenotic lesion and the patient was discharged after experiencing no further events. However, 3 months later, the patient was readmitted due to an ACS and he was treated with crush stenting with a DES. The patient mentioned at the beginning of the presentation returned 3 months later with restenosis and received a stent of the left main. This patient was enrolled into the RESERVOIR clinical trial and received an AES.
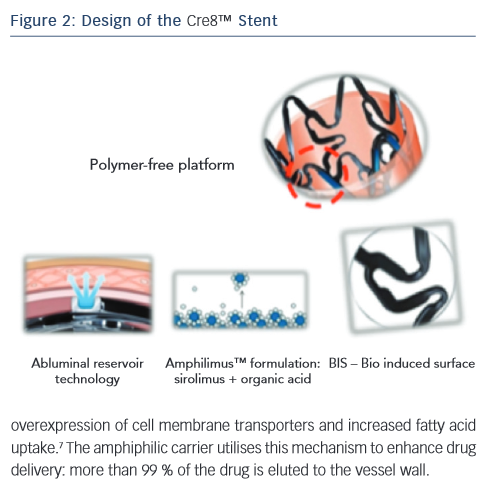
Dr Romaguera considered the causes of DES failure. Polymer-related inflammation is an important cause (see Figure 2). In addition, resistance to mammalian target of rapamycin (mTOR) inhibition is seen in patients with DM: a tenfold higher concentration of an mTOR inhibitor is needed in the diabetic cell to achieve similar inhibition to that seen in a non-diabetic one.6 The Cre8™ AES has unique features, making it well suited to patients with diabetes (see Figure 2). Among these, the amphiphilic carrier, which is a fatty acid, is a major component. Fatty acids play a main role in DM; cardiac cells cannot use glucose for energy generation as they are forced to utilise fatty acids. There is therefore an overexpression of cell membrane transporters and increased fatty acid uptake.7 The amphiphilic carrier utilises this mechanism to enhance drug delivery: more than 99 % of the drug is eluted to the vessel wall.
Dr Romaguera concluded by describing DM as the Achilles heel of interventional cardiology. Around 30 % of patients present with DM, and these patients need different DES characteristics, leading to the concept of a DES for patients with diabetes. The Cre8™ stent may fulfill this need as it has demonstrated unique efficacy in this population. However, it is important to remember that patients with DM will remain at high risk of adverse events due to disease progression in non-stented segments.
Percutaneous Coronary Intervention in a Patient with Dual Antiplatelet Therapy Constraints – Procedure and Clinical Outcome
Pieter Stella of Utrecht, The Netherlands, began by discussing PCI in the subsets of patients who have DAPT constraints: these include patients receiving oral anticoagulants (OACs), aged over 80 years, with previous bleeding events, with scheduled and urgent surgery and those non-compliant to medication.
The first factor associated with patients taking OACs is peri-procedural management during PCI. The choice of vascular access site is important, as is anticoagulant and antiplatelet management. The second factor is long-term management, where the risk of bleeding must be balanced against the risk of stent thrombosis. Factors that should be taken into consideration include bleeding risk, stroke risk (assessed by the presence of: congestive heart failure, hypertension, age ≥75 years, DM [CHADS2] score), whether or not the patient presents with ACS and the choice between BMS and DES. Age is also an important consideration; in octogenarians, the use of DES is associated with a lower cumulative death or MI rate (15.1 %) than with BMS (17.7 %).8
The duration of DAPT has been the subject of considerable recent debate. A recent meta-analysis of 10 randomised controlled trials (RCTs) and 31,666 patients investigated mortality with extended duration DAPT. Although treatment with DAPT beyond 1 year after DES implantation reduces MI and stent thrombosis, it was associated with increased mortality because of a 49 % increased risk of non-cardiovascular mortality, which is not offset by a reduction in cardiac mortality. It is worth mentioning that this could have been chance finding. In addition, prolonged DAPT has been associated with a 72 % risk of major bleeding.9 Therefore extended duration DAPT is not routinely recommended following PCI with stent implantation, but without DAPT, the risk of stent thrombosis increases.
The principal causative factors of DES stent thrombosis are summarised in Figure 3.10 Of these, around 25 % are procedural-related issues, 36 % are DES safety-related issues and 34 % are DES efficacy-related issues. In a case-controlled study of 54 cases and 35 controls, the presence of uncovered stent struts was associated with stent thrombosis after DES: with a RUTTS score exceeding 30, stent thrombosis was seen in 21.6 % of cases versus none in cases with a RUTTS score less than 30.11 In terms of DES efficacy issues related to stent restenosis, a RCT showed that EES were comparable to sirolimus-eluting stents (SES) in terms of overall clinical efficacy and safety.12 Therefore, optimising the choice of DES can reduce stent thrombosis by approximately 70 %.
The Cre8™ DES features a polymer-free platform to reduce the risk of inflammation associated with durable polymers and the breakdown products of absorbable biopolymers (see Figure 2). A drug-delivery system, abluminal reservoir technology, utilises a formulation of sirolimus and a fatty acid, amphiphilic carrier. Sirolimus, an mTor inhibitor, is an immunosuppressant with antiproliferative and antimicrobial activity. In addition, it inhibits inflammatory cell activities and has high potency. The organic acid maintains sustained drug delivery; fatty acids are used to improve the transdermal delivery of numerous drugs.13 The fatty acid also enhances drug stability, as well as increasing the bioavailability of the drug; cardiac fatty acid uptake is doubled in diabetic mouse models.14 This results in an increased homogeneous drug distribution in diabetic cardiac cells. In addition, the bio inducer surface, a second-generation integral pure carbon coating, is designed to accelerate stent endothelialisation and strut coverage, thus reducing the risk of thrombosis. The Cre8™ DES is polymer-free, eliminating the disadvantages associated with durable polymers or their breakdown products.
To end the presentation, Professor Stella discussed his experience with implantation of the Cre8™ stent in the University Medical Centre, Utrecht. To date, 786 implantations have been performed, 372 with ACS and 414 with stable disease. All stable elective cases were treated with life-long aspirin and clopidogrel for 3 months; all ACS cases were treated with 12 months of DAPT. Outcomes to date have been excellent. In patients presenting with stable disease: at 6 months, stent thrombosis was seen in only 0.2 % of patients and TLR in 1.2 %. These findings have led to a new study design; an investigator-initiated randomised two-centre study, RECre8, which is currently enrolling, aims to recruit around 1,530 patients and is designed to compare the Resolute Integrity DES (Medtronic) versus Cre8™. Participants will be given 1 month DAPT in elective PCI and 12 months DAPT in ACS. Clinical follow up will be at 12 months and 3 years and will assess all MACE.
To end the presentation, Professor Stella presented a case of a 78-year-old man admitted for colon cancer, who suffered an ACS; an ECG showed inferolateral MI. Potential strategies included plain old balloon angioplasty (POBA), drug-eluting balloon (DEB) and implantation of a BMS or DES. The patient was treated with Cre8™ DES implantation and 4 weeks DAPT. At 4 weeks, OCT showed that all the struts were covered; therefore, the patient was referred for surgery and DAPT therapy was stopped. Six weeks later, an untreated residual stenosis was stented. The patient was doing well at clinical follow-up.
In conclusion, the Cre8™ polymer-free DES combines excellent efficacy with the safety associated with BMS, and offers the potential for reduced duration of DAPT. The ReCre8 study will further study the efficacy and safety of the Cre8™ stent with further reduced DAPT.
Summary and Concluding Remarks
Patients with DM often present with multivessel disease and complex lesions. The polymer-free technology of the Cre8™ stent makes it a good choice in patients with diabetes. Clinical studies to date have demonstrated excellent outcomes in this important patient subgroup. The live case demonstrated the advantages of the Cre8™, which include minimising time taking DAPT, an important consideration for patients who are also receiving oral anticoagulation.