Mitral regurgitation (MR) is the most common valvular disease in the developed world, affecting approximately 24.2 million people worldwide and more than 2 million adults in the US.1–3 It is projected to double by 2030.4
MR is divided into two subsets: primary MR, also referred to as degenerative MR (DMR), and secondary MR, that is, functional MR (FMR). DMR describes a diseased valve apparatus, including the leaflets or chordae. DMR aetiologies include myxomatous change, rheumatic disease, infective endocarditis, collagen disorders, congenital malformations, trauma, and spontaneous rupture of chordae.
In contrast, FMR describes atrial or ventricular factors leading to poor coaptation of an otherwise non-diseased valve and affects up to 16,250 per million individuals.4 Treatment of FMR has been historically limited and less successful than DMR. Rapid development of diverse transcatheter options may provide solutions for patients who previously had none.
This review focuses on FMR, presenting the evolving data for transcatheter edge-to-edge repair (TEER), examining current guidelines for patient selection, and surveying the expansion of transcatheter mitral annuloplasty and transcatheter mitral valve replacement (TMVR) techniques, currently being evaluated in investigational trials.
Ventricular FMR and Pathophysiology
FMR in the context of left ventricular (LV) dysfunction can be due to ischaemic, dilated, or hypertrophic cardiomyopathy, causing either annular dilation or tension on the chordae tendineae. FMR after MI occurs in 20–25% of patients and up to 50% of heart failure patients.5 An imbalance of increased tethering and decreased valve coaptation results in regurgitation. Tethering forces relate to LV dilation and papillary muscle displacement, as in dilated cardiomyopathy. In contrast, closing forces are influenced by alterations in LV contractility and synchronicity, as seen in ischaemia and hypertrophic cardiomyopathy.
Atrial FMR and Pathophysiology
Atrial FMR is most commonly seen in patients with AF and patients with heart failure with preserved ejection fraction (HFpEF). Gertz et al. found a 7% prevalence in patients referred for AF ablation.6 Multiple studies have shown isolated mitral annular dilation as the mechanism of malcoaptation of the valve leaflets in patients with AF, independent of LV dilation or hypertrophy.6 A substantial proportion of patients with HFpEF have AF during the disease course, suggesting a shared pathophysiological mechanism.7
Increased left atrial pressures, seen in both AF and HFpEF, may cause myocyte remodelling leading to atrial fibrosis, annular thickening and electrical remodelling.7 Patients with AF and HFpEF have been shown to have greater left atria remodelling, exertional intolerance and worse overall outcomes.
Diagnosis
The initial diagnosis of MR can be made on transthoracic echocardiography (TTE). Further confirmation of severity and delineation of the aetiology is determined with trans-oesophageal echocardiography (TOE) or occasionally MRI and CT. The leaflets in FMR may be disease free, or mildly thickened and fibrotic with restricted motion and malcoaptation. Leaflet tethering may be symmetric, as seen in dilated cardiomyopathy, or asymmetric, more commonly seen with ischaemic disease. Tethering can be measured using the distance from the coaptation point to the annular plane (tenting height) and the area between leaflets to the annular plane (tenting area). Competent mitral valve leaflets are characterised by a sufficient coaptation surface of approximately 8–10 mm. Colour flow Doppler is used to quantify the severity of the MR jet. Three components are typically measured: proximal jet width, jet area, and flow convergence.
The 2017 American Society of Echocardiography defines four severity categories of MR: grade I, defined as effective regurgitant orifice area (EROA) <0.2 cm2 , regurgitant volume <30 ml and regurgitant fraction <30%; grade II, EROA 0.2–0.29 cm2 , regurgitant volume 30–44 ml and regurgitant fraction 30–39%; grade III, EROA 0.30–0.39 cm2 , regurgitant volume 45–59 ml and regurgitant fraction 40–49%; and grade IV, EROA ≥0.4 cm2 , regurgitant volume ≥60 ml and regurgitant fraction ≥50%.8 The 2020 American College of Cardiology/American Heart Association (ACC/ AHA) guidelines and 2010 European Association of Echocardiography definition of severe MR equates to the American Society of Echocardiography grade IV MR.9,10
Carpentier Classification for Mitral Regurgitation
MR is often classified based on leaflet motion, as described initially by Dr Alain Carpentier.11 MR associated with normal leaflet motion but loss of valve competence from annular and/or ventricular dilatation is categorised as type I and is a form of FMR. This category is often seen in patients with chronic AF and dilated left atria or annular and ventricular dilatation from non-ischaemic/idiopathic dilated cardiomyopathies, in which concentric dilatation of the ventricle is frequent. Type II is associated with excessive leaflet motion seen with papillary muscle rupture, chordal rupture, or redundant chordae leading to leaflet prolapse or flail segments and is a form of DMR. Type III describes restricted leaflet motion with two subtypes: IIIa, associated with leaflet motion restricted in both systole and diastole; and IIIb, associated with leaflet motion restricted only in systole. Type IIIa may result from rheumatic heart disease or systemic lupus erythematosus disease states and is a form of DMR. Type IIIb is often associated with posterolateral or inferior MIs from atherosclerotic lesions in the left circumflex or the posterior descending artery of the right coronary artery and is a form of FMR.
Transcatheter Mitral Repair
Transcatheter Edge-to-Edge Repair
Historically, neither surgical replacement nor repair of the mitral valve has been shown to improve survival in the FMR patient population. Edge-toedge repair of FMR was first performed surgically by Alfieri.12 Termed the ‘bow-tie technique’, the Alfieri stitch sutures opposing leaflets, creating a double-orifice mitral valve. This technique was frequently used in conjunction with an undersized mitral annuloplasty to reduce the size of the annulus and thus reduce tension on the Alfieri stitch at the level of the leaflet to restore coaptation. Mid-term and long-term outcomes were suboptimal if an annuloplasty was not included.13–15
The first known attempts at TEER were reported by St Goar using E-Valve, a clip-like device with two articulating arms, each capturing the free edge of one of the mitral valve leaflets at the site of regurgitation. The captured leaflet edges were drawn together and locked within the clip, forming a permanent tissue bridge between the leaflets at the site of regurgitation.16–18 The device was later refined and released as the MitraClip system (Abbott).
The MOBIUS system (Edwards Lifesciences) was a similar TEER device with an 11 Fr transseptal catheter and suction mechanism at the tip to capture the mitral valve leaflets.19 A suture was passed through each leaflet with a needle, drawn together, and stapled to create the repair. Although effective in preclinical studies, the technology failed to yield adequate functional outcomes in the MILANO I and II clinical studies, leading to its abandonment.19 Edwards Lifesciences has more recently received Food and Drug Administration (FDA) approval for the TEER PASCAL system, which grasps individual leaflets like the MitraClip system but has a distinct spacer between the leaflets designed to decrease tension on the leaflet tissue.20 Instead of forming an actual tissue bridge, the PASCAL system draws the leaflets onto a spacer and reduces the extent of leaflet tethering needed to approximate the leaflets. V-Clamp is a trans-apical TEER system developed by Shanghai Hongyu Medical Technology, which grasps the leaflets between fixed arms of a linearly translatable clip, after which the clip is articulated to lock the leaflets together. Only preclinical safety data are currently available, with the device presently marketed for pet canines; clinical translation is pending.21
TEER may carry advantages beyond an Alfieri stitch. There is a common misconception from the acronym that TEER aligns the edges of the mitral leaflets when, in fact, extensive tissue investment occurs within the devices at the coaptation plane, not at the actual edge of the leaflets. Additional leaflet tissue grasped in the devices may contribute to better efficacy than the Alfieri stitch. TEER enables real-time assessment of MR jet reduction in a beating heart, which aids in adaptable, correct placement of the device or multiple devices, even if the main jet is eccentric. In contrast, the Alfieri stitch is limited by dependence on preoperative MR jet assessment and is typically placed at the centre of the A2 and P2 segments of the mitral valve.
Clinical Evidence for TEER
MitraClip was the first commercially approved TEER device for the treatment of MR. The technology was approved first in Europe based on the results of the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) I and II trials. In EVEREST I (n=107 patients with MR: DMR n=84, FMR n=23), 74% met the criteria for acute procedural success, and 90% were alive at 3 years.22–24 This led to a CE (Conformité Européenne) mark in Europe for the commercial use of MitraClip for both DMR and FMR. The EVEREST II trial (n=279, DMR 73% and FMR 27%) was designed as a randomised controlled trial to compare the safety and effectiveness of MitraClip to that of surgical repair or replacement.25–28 Subgroup analysis based on aetiological classification demonstrated a risk–benefit ratio favouring MitraClip over surgery for the treatment of FMR. In 2013, MitraClip was approved by the FDA for treating 3–4+ DMR in patients at prohibitive risk for mitral valve surgery who could benefit from the reduction of MR.
After approval, MitraClip usage was captured in both US and European registries. In a Society of Thoracic Surgery/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry study spanning nearly 2 years, 2,952 patients were treated, of whom 85.9% had degenerative aetiology, 8.6% had functional aetiology, and the remaining 5.5% had mixed lesions.29 Procedure success was 91.8%, and in-hospital death was 2.7%. The data demonstrated improved outcomes but without change in survival or hospitalisations. Mortality remained at 24.7% and 31.2% at 12 months after MitraClip in DMR and FMR patients, respectively. In the European ACCESS-EU post-approval study of MitraClip in 567 patients, 69.3% had FMR, with most having MR grade >3 or >4 at baseline and New York Heart Association (NYHA) functional classes III or IV. A majority (92%) of the patients with FMR achieved MR reduction to 2+ or less at discharge, and only 11 patients with FMR (2.8%) died within 30 days, four of whom died of cardiac causes.30 However, long-term survival benefits remain unclear.
The promising results of TEER registry data stimulated the design of two landmark randomised controlled trials, MITRA-FR (the Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients with Severe Secondary Mitral Regurgitation) and COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Regurgitation). Both trials compared MitraClip repair of FMR and guideline-directed medical therapy (GDMT) against medical therapy alone. MITRA-FR, sponsored by the French Ministry of Health and Research National Program and Abbott Vascular, recruited 304 patients with FMR due to LV dysfunction with an ejection fraction (EF) ranging from 15% to 40%, NYHA classes II–IV, and at least one heart failure hospitalisation in the past 12 months.31,32 MR severity with an EROA >20 mm2 , or regurgitant volume >30 ml/beat, while on heart failure medications were entry criteria. Patients were randomised to receive either MitraClip with medical therapy or medical therapy alone. The primary endpoint was freedom from death or hospitalisation at 12 months. COAPT, sponsored by Abbott Vascular and initiated in the US, recruited 614 patients with FMR from LV dysfunction and symptomatic heart failure with 3 or 4+ MR grade per American Society of Echocardiography guidelines, defined as EROA >30 mm2 and/or regurgitant volume >45 ml, and symptomatic despite maximally tolerated GDMT or use of cardiac resynchronisation therapy. The primary outcome was all heart failure hospitalisations at 24 months.33
The outcomes of the MITRA-FR and COAPT trials were distinctly different (Supplementary Material Table 1). In the MITRA-FR trial, there was no difference between the intervention versus control for the primary composite outcome (54.6% versus 51.3% respectively), mortality (24.3% versus 22.4%), or the rate of unplanned heart failure hospitalisation (48.7% versus 47.4%). Thus, this trial concluded that although MitraClip is safe and effective in reducing FMR, it does not improve prognosis compared with GDMT. In contrast, the COAPT trial reported overwhelmingly positive results, with the primary endpoint of an annualised rate of all hospitalisations for heart failure within 2 years of 35.8% per patient-year in the intervention group as compared with 67.9% per patient-year in the control group (p<0.001). Death from any cause occurred in 29.1% with MitraClip versus 46.1% in the control group (p<0.001). The COAPT investigators concluded that in patients with heart failure and moderateto-severe or severe FMR who remained symptomatic despite optimal GDMT, MitraClip reduces hospitalisation rates and all-cause mortality.
Several factors may explain the divergent results. The recruitment in COAPT was more selective and prolonged. The COAPT trial excluded a more significant proportion of patients initially screened (66% versus 33% in the MITRA-FR trial), with the highest enrolment centre enrolling only 46 patients in 5 years. MITRA-FR used the European definition of severe MR, EROA >20 mm2 , regurgitant volume >30 ml/beat (moderate MR by AHA guidelines), whereas COAPT used the US definitions (EROA >30 mm2 , regurgitant volume >45 ml/beat). The COAPT trial enrolled patients with more severe MR, EROA 41 versus 31 mm2 , but lower LV dysfunction, as demonstrated by a lower LV end-diastolic volume (101 versus 135 ml/m2 ). It has been suggested that the MITRA-FR patients had more advanced ventricular remodelling than those in the COAPT trial and were less likely to benefit from MitraClip. Imaging parameters such as LV strain have been suggested to identify patients more likely to benefit from percutaneous mitral valve intervention, but have not been conclusive.34 For those patients who did pass conservative screening, COAPT operators were perhaps more aggressive, demonstrating superior procedural success rates compared with MITRA-FR, with lower rates of postprocedural MR 3+ (5% versus 9%) and 12-month residual MR 3+ (5% versus 17%). In addition, GDMT was rigorously optimised before recruitment in COAPT and was stringently monitored compared with MITRA-FR, where medical management was not subject to scrutiny.
Considering the results of both the MITRA-FR and COAPT trials, it appears reasonable to conclude that the MitraClip procedure reduces heart failure hospitalisation and mortality in patients meeting the following criteria: moderate-to-severe secondary MR defined as EROA ≥30 mm2 and/or regurgitant volume >45 ml; LV ejection fraction (LVEF) 20–50% and LV end-systolic diameter (LVESD) <70 mm; and persistent heart failure symptoms defined as NYHA class ≥II despite optimal GDMT.
PASCAL
The PASCAL mitral valve repair system (Edwards Lifesciences) uses a transseptal approach and edge-to-edge repair technique combined with a central spacer to fill the regurgitant orifice area. It consists of two paddles and clasps to grasp each leaflet independently and enables optimal positioning. The delivery system includes a 22 Fr guide with a steerable catheter. The CLASP study (Edwards PASCAL Transcatheter Mitral Valve Repair System Study) enrolled 62 patients with grade 3+ or 4+ MR and showed the feasibility and acceptable safety of the PASCAL system in the treatment of severe MR at 30 days.35 One-year follow-up demonstrated a robust sustained reduction in MR in 109 patients (67% functional, 33% degenerative) accompanied by a low complication rate, high survival (92%), and significant improvements in functional status and quality of life (88% freedom from hospitalisation) at 1 year.36 PASCAL received a CE mark in 2019 and FDA approval in September 2022.37
The CLASP IID/IIF (Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial) is an ongoing prospective multicentre randomised controlled pivotal trial that aims to establish the safety and effectiveness of the PASCAL mitral repair system compared with MitraClip in patients with DMR deemed at prohibitive risk for mitral valve surgery and patients with FMR optimised on GDMT. The study is enrolling, with an estimated completion date of January 2028 (NCT03706833).
Patient Selection for TEER
Anatomical factors that may make TEER challenging:
- small mitral valve orifice area <3 cm2 with gradient >5 mmHg at baseline;
- rheumatic disease or similar anatomy with calcified chords, thickened immobile leaflets, especially immobile posterior leaflet;
- severe Barlow’s anatomy;
- dominant jet near the lateral commissure with taut chord; severe right ventricular failure;
- flail leaflet with a width of the flail segment >15 mm and flail gap of >10 mm; and
- length of posterior leaflet <7 mm.
The potential contraindications for TEER are as follows:
- severely calcified valve leaflets in the grasping area;
- perforated mitral leaflets or clefts, lack of primary and secondary chordal support;
- massive EROA with jet across the entire line of coaptation with tented flattened leaflets;
- inability to tolerate procedural anticoagulation or post-procedural antiplatelet regimens;
- active endocarditis; and
- evidence of intracardiac, vena cava, or femoral venous thrombus.38–41
TEER Device Design and Selection
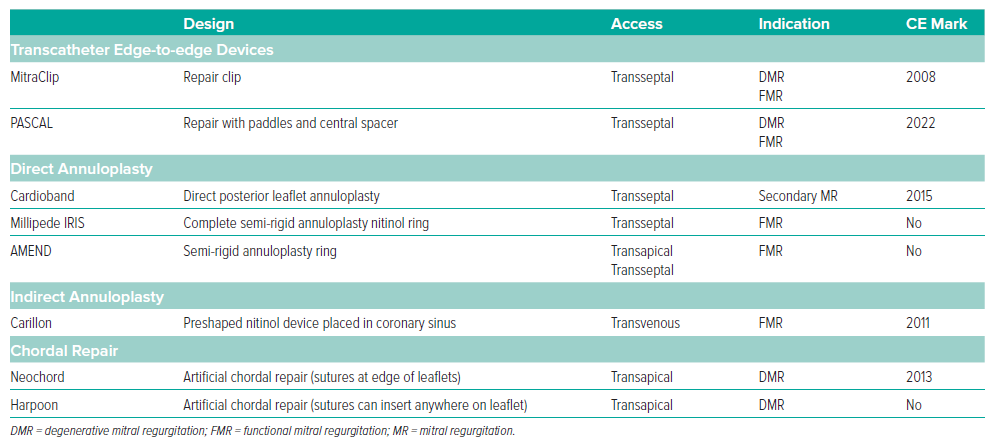
The indications for selection of TEER device and mitral valve repair device are listed in Tables 1 and 2.
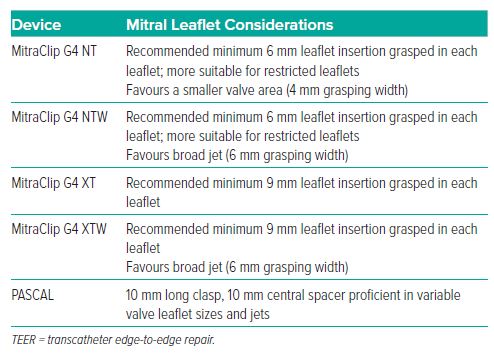
MitraClip is currently on its 4th generation (G4), which was approved by the FDA in 2019. A small retrospective study showed a 96% reduction in MR at 30 days with the new-generation devices.40 Notable features of the G4 system include the ability to monitor left atrial pressure through the delivery system, independent leaflet grasping, and various device sizes.40 The G4 clips come in four sizes: NT, NTW, XT and XTW.42 The NT and NTW have a shorter grasping span, 17 mm at 120° fully open, requiring a recommended 6 mm of leaflet insertion for a successful grasp. The XT and XTW clips have a longer span, 22 mm at 120°, requiring 9 mm of leaflet insertion for successful grasp. The ‘W’ in NTW and XTW stands for wide; the wider width is 6 mm; the NT and XT clips are 4 mm wide.
Clip sizing strategies vary by institution but, in general, the shorterspanning NT and NTW clips may be favoured for patients at risk for excessive leaflet tension (i.e. posterior tethering in FMR, small valvular area, and mitral annular calcification). Shorter clips coapt less leaflet tissue with perhaps less risk of excessive tension, worsening FMR, and future risk of leaflet tears. The XT and XTW longer grasping wingspan may be favoured for valves with redundant leaflet tissue, significant coaptation gaps, and flail segments. The choice of clip needs to balance the width of the jet to be closed against the occasionally tight arrangements of the underlying chordae tendineae to be traversed during deployment. Independent, reversible leaflet grippers on the G4 system add a level of procedural safety and flexibility to grasp one leaflet at a time, reposition, and customise the deployment based on the dynamic anatomy. Although some anatomy may allow only a single clip, the goal should be the greatest achievable reduction in MR. Multiple clips may be placed to accomplish this goal, provided that the transvalvular gradients and left atrial pressures remain in acceptable ranges. Deploying different size clips onto the same valve is possible, accounting for the theoretical possibility of varying tension dynamics on the leaflet tissue. Positioning a second or third clip must balance the possibility of dislodging a prior clip against leaving narrow haemolysing jet gaps.
Similarly, the PASCAL system has undergone modification with narrower paddles (PASCAL Ace), reducing the size of the implant. Head-to-head trials are ongoing to compare PASCAL and MitraClip. In practice, there are multiple institutional strategies to optimise clip selection tailored to the patient’s anatomy.
Transcatheter Mitral Annuloplasty
Simulating surgical annuloplasty, these devices are designed to reduce the annular dimension and achieve leaflet coaptation. Implantation can be direct or indirect regarding the relationship to the mitral annulus (Table 2). The direct mitral annuloplasty methods include Cardioband (Edwards Lifesciences), Millipede (Boston Scientific, no longer under investigation), and AMEND (Valcare). Direct mitral annuloplasty has proven challenging, and no device has progressed in development through a pivotal trial. However, indirect mitral annuloplasty such as the Carillon Mitral Contour System (Cardiac Dimensions) and the ARTO system (MVRx, also no longer under investigation) are based on the parallel relationship of the coronary sinus to the mitral annulus.
Cardioband Mitral Annuloplasty System
Cardioband received a CE mark in 2016. The transseptal system consists of screw anchors covered by a polyester sleeve. The first anchor is placed at the anterior and lateral sides of the mitral annulus. The remaining anchors are placed at the mitral annulus up to the medial side. A contraction wire follows the same path, attaching to a spool that, once activated, cinches the Cardioband device, reducing the annular diameter. The randomised controlled trial in the US was paused during the COVID-19 pandemic, and the device is being reintroduced as a tricuspid annuloplasty.
AMEND
The AMEND system is a semi-rigid D-shaped mitral annuloplasty ring directly attached to the mitral annulus in a transapical or transseptal approach. The device has a series of barbed anchors that enable it to be anchored first on the posterior annulus, and then it can be pulled and anchored anteriorly. The company reported its first successful human implantation of the ring via transseptal delivery system at the Schulich Heart Centre in Toronto in 2022.43
Carillon Mitral Contour System
The Carillon Mitral Contour System (Cardiac Dimensions) was CE marked in 2011. It consists of a sizing catheter, a delivery catheter and the Carillon XE implant. The sizing is based on the dimensions of the coronary sinus and greater cardiac vein. The CarillonXE2 implant has a distal anchor (positioned in the greater cardiac vein), proximal anchor (positioned in the coronary sinus), ribbon connector (joining the anchors), and proximal and distal crimp tubes, which are deployed and secured under appropriate tension in the coronary sinus. In the randomised sham-controlled REDUCE FMR trial with 120 patients, the Carillon device was shown to significantly reduce MR volumes (−7.1 ml/beat versus +3.3 ml/beat in the sham-control group) and LV volumes in symptomatic patients with FMR receiving GDMT.44 Recently published 5-year follow-up data also showed durable functional improvement and favourable 5-year survival rates.45 A randomised trial comparing this device to GDMT in 352 FMR patients is underway at 75 sites in Europe and the US (NCT03142152). Limitations include the risk of injury to the left circumflex artery by the distal anchor, the distance of the coronary sinus from the mitral annulus, and the inability to place the device in patients with coronary sinus pacemaker leads.
Overall, the clinical application of annuloplasty devices has not been widespread due to procedural challenges and device complexity. Other techniques, such as transcatheter mitral valve chordal repair, including Neochord and the Harpoon Mitral Valve Repair System, are undergoing randomised trials to treat severe DMR. It remains to be seen whether the repair devices under investigation will prove useful as standalone therapies or in combination to mimic surgical repair.
Treatment Guidelines: US versus European
With the development of transcatheter options, the decision to replace or repair a mitral valve has become challenging, and the guidelines continue to adapt as more clinical data accrue and occasionally conflict (Table 3). As described above, COAPT demonstrated improvement in heart failure hospitalisation, survival, symptoms and quality of life in patients treated with TEER with MitraClip.33 In contrast, MITRA-FR reported no benefit of TEER in reducing the composite endpoint of death or hospitalisation compared with medical therapy.32
These two randomised controlled trials impacted the level of evidence and recommendations regarding the use of TEER in FMR. Both studies evaluated TEER as a minimally invasive treatment option in patients with severe FMR who were symptomatic despite optimal medical therapy and deemed not operable by the Heart Team.32,33 COAPT included patients with more advanced MR and less LV enlargement compared with MITRAFR.32,33 Survival and hospitalisation were significantly better in COAPT, not demonstrated in MITRA-FR.
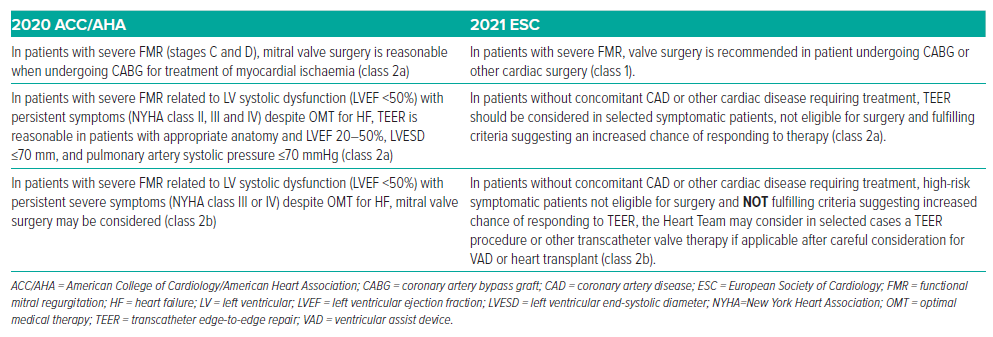
The 2021 European Society of Cardiology (ESC) guidelines recommend (class 1) that patients with severe FMR who are scheduled for coronary artery bypass grafting or other cardiac operations should undergo mitral surgery.46 In contrast, the 2020 ACC/AHA guidelines rate this as a class 2a recommendation.9 The prior 2017 ESC guideline requirement for LVEF >30% was dropped in the updated 2020 ESC recommendations.47
The 2020 ACC/AHA guidelines now suggest TEER as a class 2a indication for patients who meet the criteria outlined in the COAPT trial (LVEF 20– 50%, LVESD ≤70 mm, pulmonary artery systolic pressure ≤70 mmHg).9 In patients with severe FMR and no other indications for intervention, there is a class 2b indication for TEER per the ESC guidelines.46
ESC guidelines have revised the recommendation for high-risk symptomatic patients who are ineligible for surgery and who do not meet criteria suggesting an increased chance of responding to TEER. In selected cases, after careful consideration for a ventricular assist device or heart transplant, the Heart Team may consider a TEER procedure or other transcatheter valve therapy (class 2b) irrespective of LVEF (previously requiring LVEF <30% per 2017 ESC).46 The ACC/AHA guidelines do not include any recommendations for this group of patients. As a new recommendation, the ESC guidelines recommend TEER in patients with severe FMR who are symptomatic after transcatheter aortic valve replacement (TAVR) or percutaneous coronary intervention (class 2a).9,46
The guidelines do not yet address transcatheter FMR treatments outside of TEER, given that modalities such as annuloplasty and TMVR are still in clinical trials.
Transcatheter Mitral Valve Replacement
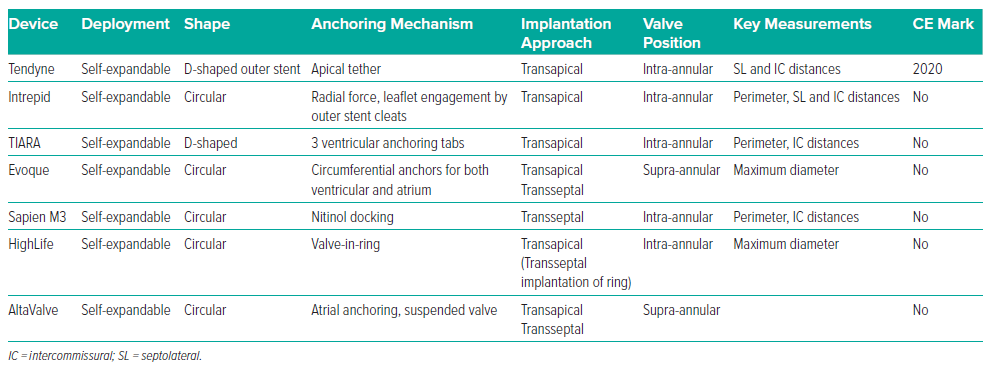
Despite the results, TEER remains limited in its applicability. The only FDAapproved TEER device for FMR is MitraClip; PASCAL currently has FDA approval only for DMR. Both TEER devices are restricted to specific anatomies and may still leave patients with moderate or greater residual MR with continued risk of morbidity and mortality. A more durable solution may be offered by TMVR (Table 4).48
Given the dynamic nature of mitral valve anatomy, careful TMVR procedural planning is required using cardiac CT analysis. Specific measurements and visualisation of valve anatomy aid in choosing the appropriate device to decrease the risk of complications such as LV outflow tract obstruction (LVOTO), paravalvular leak, embolisation and patient–prosthesis mismatch. Typical measurements include intercommissural, septal-to-lateral, and trigone-to-trigone distances and mitral annular perimeter. The LV outflow tract (LVOT) is assessed for a long anterior mitral leaflet or septal hypertrophy and LVOTO risk after valve deployment. The extent and location of mitral annular calcifications should also be ascertained. Asymmetrical annular calcification may interfere with successful device seating, leading to a higher risk of embolisation or paravalvular leak. Calcification protruding from the anterior leaflet poses a risk if displaced.49 CT is also used to assess cardiac structures at risk of injury during the procedure, such as the left circumflex artery and the coronary sinus. CT aids in determining the adequacy of the chest wall for transapical access, which TTE and TOE cannot provide. Last, CT may be combined with echocardiography to assess the atrial septum for transseptal delivery.
Tendyne
The Tendyne MV system (Abbott Structural) was the first approved device in Europe for transcatheter implantation in a native mitral valve. It is a selfexpanding trileaflet porcine pericardial valve mounted on a nitinol frame implanted via the transapical approach; it can be repositioned and retrieved. After deployment, the valve remains tethered to the LV apex closure pad via a polyethylene tether. Varying patient annuli can be accommodated by a single inner valve size paired with multiple outer frame sizes and a large effective orifice area (>3.0 cm2 ). The device received the CE mark in January 2020 after data from the early feasibility trial of 100 patients demonstrated 97% technical success, reduction to none or trivial MR in 98.8% of patients, and 72.4% overall 1-year survival.50,51 The 2-year findings reinforced the 1-year observations showing a decrease in heart failure hospitalisations, decreased compared with pre-Tendyne implantation (0.51 versus 1.30 events per year; p<0.0001); however, the rate of all-cause mortality remained high.52 The pivotal SUMMIT trial (NCT03433274) randomising patients to Tendyne or MitraClip was stopped due to slow enrolment.
Intrepid
The Intrepid Valve system (Medtronic) is a self-expanding trileaflet bovine pericardial valve mounted on a nitinol stent frame. Initially implanted via the transapical approach, the transseptal delivery system is undergoing early feasibility studies but initially required a surgical cut down due to the large sheath size.53 The atrial portion of the valve is large and is designed to seat and seal the device in the native annulus. Initial data from a cohort of 50 patients showed successful transapical device placement in 96% of patients with a 30-day mortality of 14%.54 Transseptal early feasibility studies have shown no death, stroke, or reintervention at 30 days, and all patients who underwent implantation had none or trace valvular or paravalvular MR.53 A pivotal US-based trial, Apollo (NCT03242642), is under way. Initially, the trial was designed for patients with symptomatic, severe MR randomised to either the Intrepid TMVR system or traditional surgery, with a primary composite endpoint of all-cause mortality, stroke, re-intervention, and cardiovascular hospitalisation at 1 year. However, the Apollo trial design was changed to randomise Intrepid TMVR versus TEER. Further additions to the trial include a mitral annular calcification registry, international sites, and the new 29 Fr transseptal delivery system.
TIARA
The Tiara transcatheter mitral valve (Neovasc) is a trileaflet bovine pericardial valve mounted on a nitinol frame. The self-expanding valve is implanted via a transapical approach. This device has a large atrial skirt to seat the device and minimise paravalvular leak. It uses three ventricular tabs that anchor into the LV myocardium. Early feasibility trials, TIARA-I (NCT02276547) and TIARA-II (NCT03039855), are ongoing with a transseptal system currently under development.55
Evoque Eos
The Evoque Eos (Edwards Lifesciences) valve system consists of a trileaflet bovine pericardial valve mounted on a nitinol frame. The device can be delivered transeptally and has a unique anchoring mechanism that relies on the annulus, leaflets and chords. The device’s low profile and intra-annular sealing skirt and frame serve to minimise paravalvular leak. Early results in a small group of 14 patients showed a 93% procedural success and elimination of MR in 80% of patients.56
Sapien M3
The Sapien M3 (Edwards Lifesciences) combines a modified Sapien 3 transcatheter aortic valve and a coiling nitinol docking system. M3 is a balloon-expandable bovine pericardial valve mounted on a cobaltchromium frame. The nitinol coiling system is placed around the mitral valve leaflets, and the M3 valve is placed inside this docking system. The valve is delivered via the transseptal approach. Early experience reported 87% technical success and 30-day all-cause mortality rate 2.9%.57 At 30 days, all patients had no or mild residual MR, but one had a 3+ paravalvular leak.
HighLife Valve
The HighLife valve (HighLife SAS) is a two-component system: a subannular implant creates a closed loop with a fixed perimeter around the native valve leaflets and chordae, and the prosthesis is then implanted by anchoring to the subannular component. Early experience with 15 patients showed a 30 day mortality of 20% and 1 year mortality of 27% (NCT02974881).58 No paravalvular leak was observed, but there was one documented case of LVOTO.
AltaValve
AltaValve (4C Medical Technologies) has a unique, spherical nitinol frame design with atrial anchoring and a valve suspended in the annulus. The delivery catheter comes in both transapical and transseptal systems. The implant does not rely on annular fixation with barbs or anchors and does not require oversizing at the annulus level. The supra-annular design mitigates the risk of LVOTO and preserves the native annular dimensions given that no oversizing is necessary. The device is in an early feasibility study (NCT03997305).
Other valve systems under investigation include the Cardiovalve (Cardiovalve), the Cephea Valve (Cephea Valve Technologies), the NSCI NaviGate valve (NaviGate Cardiac Structures), and the MValve (MValve).
Technical Challenges
There are many technical challenges facing TMVR system development.59 Intra-annular valves risk LVOTO by the high profile valve frame and displacement of the anterior mitral leaflet.60 The location of the mitral valve enables transfemoral–transseptal or transapical approaches, but the delivery systems are large and require a high degree of flexion if delivered transeptally. Many TMVR systems were initially developed for transapical access, with subsequent modifications made for transseptal delivery. Similar to the transapical TAVR experience, transapical TMVR is generally associated with poorer outcomes compared with the transfemoral approach and is poorly tolerated in elderly patients.61,62
Another major technical challenge for TMVR is the difficulty in anchoring the device. For TAVR, the aortic valve is calcified and rigid, enabling the bioprosthesis to anchor reliably within the fixed native annulus. Regurgitant mitral valves are generally not calcified, and the annulus is a dynamic structure throughout the phases of the cardiac cycle. Malposition, destabilisation and embolisation are potential risks because the mitral annulus is not a rigid structure. Last, TMVR is associated with postoperative heart failure, especially when done in later stages of the disease.33 Durability, comparability to open or minimally invasive surgery, and technical feasibility require further investigation.
Which Device for Which Patient?
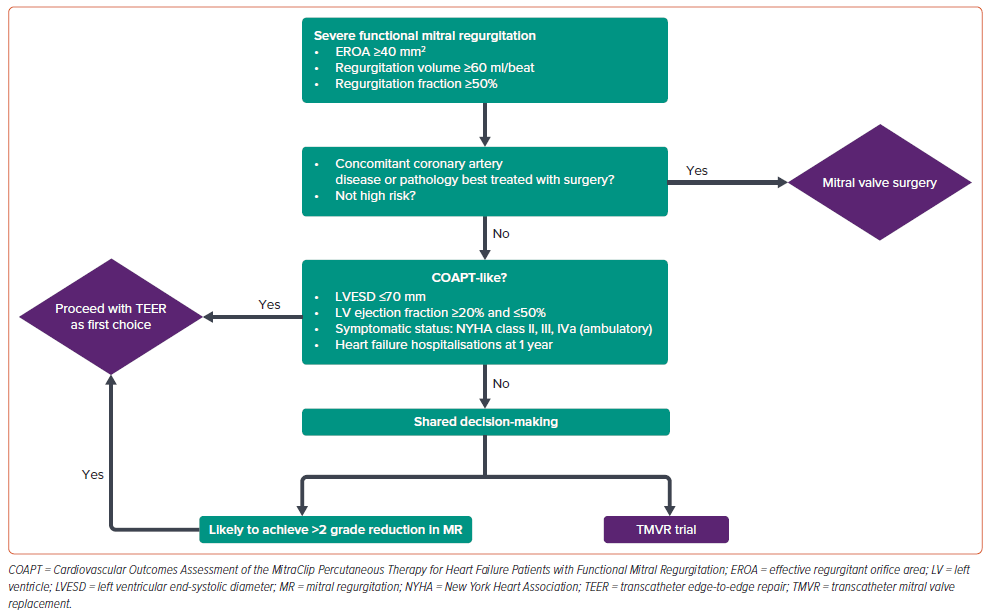
Given evolving guidelines, multiple considerations for the treatment of FMR, and limited FDA approved and CE marked transcatheter options, heart teams must consider the individual patient when determining which therapy to offer, and discuss the benefits and limitations of all available options in a manner that enables shared medical decision-making (Figure 1). Currently, there is no single best treatment for FMR.
A TEER-first approach has been implemented at our institution, using available clinical data and appreciating the limitations of the current guidelines. For patients meeting COAPT criteria and in whom it is believed a >2 MR grade reduction is likely to be achieved, TEER with MitraClip is preferred. However, the availability of PASCAL may enable further device choice based on forthcoming data and regulatory approvals. All patients are screened for participation in a TMVR trial program so that they can review the options available and consider enrolment if they qualify. For patients excluded from TMVR trials (i.e. renal failure, severe tricuspid regurgitation, anatomical factors etc.) and outside of the COAPT criteria, the decision to pursue TEER remains an option given the extremely poor result of GDMT alone. Still, it must be considered carefully given the lack of benefit seen in MITRA-FR.
With the current portfolio of TMVR devices under active investigation, the individual inclusion and exclusion criteria drive patient selection. Tendyne was the first TMVR platform to enrol patients, but the transapical approach proved poorly tolerated. With all of the TMVR devices, limited data and high screen failure rates due to clinical and anatomical exclusions currently limit broad generalisations about which TMVR device is most favourable for a given scenario. The M3 system is transseptal and offers the advantage of a docking mechanism that may draw the mitral leaflets away from the LVOT. However, its relatively ventricular position still carries a risk of LVOTO. HighLife is presumably similar, but there are limited data from which to draw conclusions. Intrepid and Evoque both offer transseptal delivery, but these systems require significant oversizing to maintain intraannular position and carry an increased risk of LVOTO. Evoque has the additional requirement of leaflet grasping by the device, which further limits anatomical eligibility. Finally, AltaValve, now also offered in a transseptal platform, mitigates the risk of LVOTO by suspending the valve in a supra-annular position in the atrium, which may preserve the native flow through the LVOT. This may be a suitable choice for patients otherwise anatomically excluded from the other studies, although it is also subject to limitations in generalisability due to clinical and anatomical exclusions.
Conclusion
In summary, a wide variety of transcatheter strategies for both repair and replacement are currently being developed (Supplementary Material Table 2) to treat patients with FMR to reduce morbidity and mortality and improve quality of life. Many devices remain in the early stages of development with limited clinical evidence. MitraClip and PASCAL are the only FDA-approved and CE-marked transcatheter edgeto-edge devices. Until further data are available, MitraClip is likely to remain the device of choice for patients with amenable leaflet and chordal anatomy due to its ease of use and strong clinical and safety data. Patients with anatomical barriers to the achievement of adequate MR reduction with current TEER technology may be better served by enrolment in ongoing novel device trials, with the choice of an annuloplasty device versus TMVR based on the consensus of the Heart Team after review of all available clinical data.