Dr O’Neill began by introducing the National Cardiogenic Shock Initiative (NCSI), a group established in April 2016 to address mechanical support management of patients with acute MI (AMI) with cardiogenic shock (CS). No protocols for mechanical support management existed at the time of NCSI inception, with great disparity in device use in the context of angioplasty. Survival after angioplasty supported with an intra-aortic balloon pump (IABP) has remained at 50% for the past 30 years. Dr O’Neill formed the Detroit Cardiogenic Shock Initiative with cardiologists in the Detroit metro area with the goal of establishing a single mechanical support protocol that would increase AMI CS survival to >70%.
NCSI is a prospective, non-randomised, single-arm, multicentre nationwide study assessing the impact of early mechanical circulatory support (MCS) in AMI CS patients treated with percutaneous coronary intervention (PCI). Partial funding is provided by Chiesi and Abiomed, but neither company had direct involvement in the study design or data analysis. More than half of the 70 sites currently participating in NCSI include non-academic, teaching or community hospitals, ensuring broad protocol applicability to AMI CS patients.
The NCSI treatment protocol consists of rapid identification of CS, catheterisation lab activation, femoral access, AMI CS confirmation and implantation of an Impella CP transvalvular support device prior to PCI within a target door-to-support time of less than 90 minutes. Angioplasty is then performed, followed by an assessment of the patient’s status by right heart catheter (RHC) monitoring of cardiac power output (CPO) to assess left heart performance and pulmonary artery pulsatilty index (PAPI) or a right atrial pressure-to-wedge pressure ratio to assess right heart performance. Higher numbers for both values indicate normal heart performance. If CPO is ≥0.6 and PAPI is >0.9, left and right heart performance are doing well, and the patient can be down-titrated off of inotropes or vasopressors and the Impella device removed within 48 hours. If CPO is <0.6, the patient is still in shock and requires further left or right heart support. If PAPI is >0.9 and CPO is <0.6, the right ventricle (RV) is considered normal and increased left ventricular (LV) support should be strongly considered. If CPO is <0.6 and PAPI is <0.9, then RV failure is possible and increased RV support should be considered.
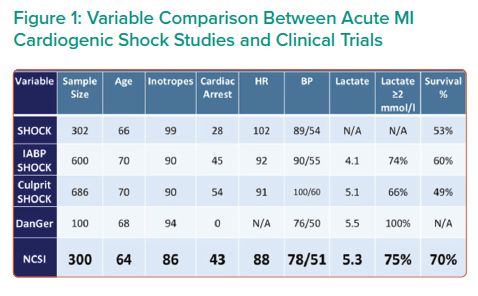
Preliminary data of 300 AMI CS patients enrolled at 57 centres demonstrated a 70% survival rate and >90% native heart recovery using the NCSI protocol.1,2 Implantation of IABP prior to referral, no AMI or anoxic brain injury were the primary reasons for patient exclusion. Comparing the NSCI result with those of five other major AMI trials and studies revealed that the NCSI survival rate of 70% is the highest reported of any modern trial or registry conducted in the US or Europe (Figure 1). Taken together, these data suggest MCS with Impella prior to PCI greatly improves patient outcomes.
The NCSI protocol improves patient survival by early identification of shock and initiation of mechanical support with Impella prior to PCI. A golden hour of care exists for AMI CS patients with a treatment delay of every 10 minutes associated with a 3.31% increase in mortality in PCI-treated patients.3 Ineffective support for AMI CS, such as primary use of IABP, can cause further treatment delays.5,6 Mechanical unloading of the heart by Impella CP pre-PCI is associated with improved AMI CS survival, and should be considered as a support option.4,7–14
Inotrope usage greatly increases mortality in AMI CS patients, with use of more than two inotropes being associated with a near doubling of the mortality rate.4,15 Increased inotrope use also correlates with poor mortality outcomes, predicted by CPO levels of <0.8.16,17 The NCSI protocol includes down-titration of inotropes to further improve survival in AMI CS patients.
Dr O’Neill concluded that improvements in mechanical support protocols, such as those implemented in the NCSI study, improve survival and native heart recovery. Best practice protocols include early identification and support of patients with cardiogenic shock, aggressive down-titration of inotropes, identification of inadequate LV support and escalation, identification and support of RV dysfunction and the systematic use of RHC to guide therapy.2,17–19 RHC provides the data critical for the optimisation of treatment. The NCSI dataset has also demonstrated functional application to the Society of Cardiovascular Angiography and Interventions shock staging system and multivessel versus culprit vessel PCI.20,21 Future studies include a large-scale randomised clinical trial of optimal MSC compared with standard of care in AMI CS.