Dr Keller opened his presentation with a clinical case to illustrate the challenges we currently face when treating patients with severe heart failure. He presented the case of a 25-year-old man with severe left ventricular (LV) dysfunction who experienced a cardiac arrest in the setting of cocaine use. After a brief arrest period, during which he received cardiopulmonary resuscitation, the patient experienced post-arrest haemodynamic instability. The patient was subsequently cannulated in the emergency department and placed on extracorporeal circulatory support via veno-arterial extracorporeal membrane oxygenation (VA-ECMO). After cannulation, an ECG showed the patient had a normal LV cavity with an LV ejection fraction (EF) of 10% and diffuse hypokinesis. However, over the following 24 hours, despite various efforts, minimal pulsatility persisted and the decision was taken to proceed with a venting strategy via placement of Impella CP to maintain forward flow. Unfortunately, formation of a large LV thrombus was detected in the apex, and the Impella could not be placed. The patient became asystolic and care was withdrawn at 48 hours.
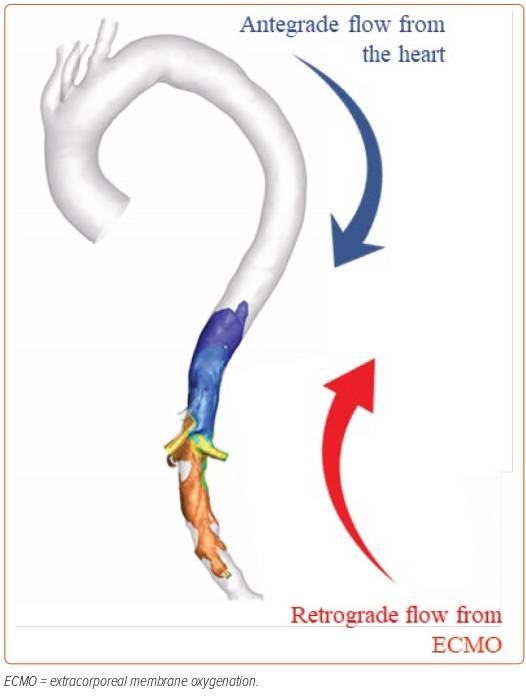
This case demonstrates the lack of accurate assessment for the need for a venting strategy: the patient experienced a catastrophic outcome even though his initial chest X-ray before ECMO showed an improvement in interstitial oedema and no signs of LV dilation. Although VA-ECMO can restore systemic perfusion, it can also adversely impact on the failing LV by increasing the afterload (Figure 1). This is accompanied by a number of other adverse effects, including increased metabolic and mechanical stress on the failing heart.
Venting strategies can be used to prevent the potential deleterious effects of VA-ECMO by mitigating the increase in the afterload. Dr Keller provided the example of two percutaneous mechanical circulatory support (MCS) devices available to vent the LV when used in combination with VA-ECMO: the intra-aortic balloon pump (IABP) and the percutaneous ventricular assist device (pVAD) that is Impella CP. However, there are limited data on how these devices function in response to the retrograde flow from the VA-ECMO.
The joint collaboration between the Massachusetts Institute of Technology (MIT) and Abiomed aimed to address this data gap by conducting a preclinical study using the porcine model of acute cardiogenic shock to perform a physiological comparison between the different MCS venting strategies. Acute cardiogenic shock was induced in six pigs via serial embolisation of the left anterior descending (LAD) or left circumflex (LCx) coronary arteries with microbeads; the pigs were then randomised to either pVAD or IABP support after undergoing peripheral VA-ECMO. The pigs met the definition of shock if two or more of the following criteria were met: mean arterial pressure <50 mmHg; LV end-diastolic pressure (LVEDP) >16 mmHg; and/or mixed venous oxygen saturation <50%.
By using LV pressure–volume loops that provide insights into ventricular physiological state and function, it was demonstrated that there was a decrease in contractility and increased LVEDP in the porcine experimental model upon the induction of shock, which is comparable to the clinical physiology of acute cardiogenic shock.
Combined VA-ECMO and IABP resulted in increased LV volume and a modest change in LVEDP. Although the IABP generated the appearance of pulsatility as the IABP cycles, caution should be exercised because this may not mean the aortic valve is opening. Conversely, combined VA-ECMO and pVAD showed a reduction in stroke work and a significant lowering of LVEDP. The continuous flow throughout the cardiac cycle achieved an increase in diastolic pressure and a decrease in systolic pressure. Further, there was an increasing degree of LVEDP drop with higher Impella Performance (P) levels (ranging from a 35% reduction at P2 to a 60% reduction at P8 with 50 ml/kg/min ECMO flow), whereas IABP had minimal impact on filling pressure (<10% change). This benefit was shown to be preserved across the different ECMO flow rates and is a titration-dependent effect.
When used together with VA-ECMO, pVAD venting significantly reduced the drivers of myocardial oxygen consumption compared with IABP venting, including LVEDP, stroke work and dP/dtmax. pVAD venting also improved the metrics of coronary and systemic perfusion compared with IABP venting, including coronary perfusion, systemic perfusion and carotid artery blood flow.
Dr Keller provided a second case example to demonstrate the application of the study findings to clinical practice. He presented the case of a 61-year-old man with coronary artery disease who had an IABP inserted preoperatively. The patient experienced a ventricular tachycardia arrest postoperatively and underwent VA-ECMO cannulation, after which the IABP was removed. On Day 2 postoperatively, the IABP was reinserted upon failure to wean off ECMO. However, the patient’s haemodynamics continued to deteriorate over the following 7 days. Upon replacement of the IABP with an Impella CP, the patient began to recover, and he was rehabilitated and discharged 2 weeks later. Dr Keller was delighted to announce that the patient was still alive 2 years later without having undergone any further hospitalisation.
In conclusion, Dr Keller highlighted the relevance of the study findings to complex clinical scenarios and the greater clinical benefits that a pVAD venting strategy, such as Impella CP, can offer over IABP venting when combined with VA-ECMO. He caveated that successful clinical use of an MCS device is dependent upon competent deployment. A clinical trial of the combined MCS approach is the next step to follow this preclinical evidence. 









