Dr Meyns shared the results of his preclinical study testing the hypothesis that extended duration of direct cardiac unloading using the high-flow Impella 5.5 pumps can initiate reverse remodelling in chronic heart failure. The goals of this study were to describe the myocardial functional, cellular and subcellular-level effects of cardiac unloading and gain insights into the optimised mode of unloading.
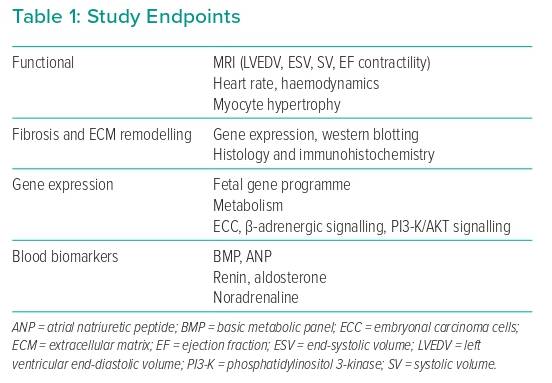
The chronic heart failure model used in the study was generated by permanent ligation of the diagonal branches, combined with ischemia–reperfusion of the mid-section of the left anterior descending artery, in sheep. This model resulted in an ischaemic cardiomyopathy phenotype with infarct, border and remote zones in the myocardium, similar to a clinical presentation of ischaemic cardiopathy after MI. Over time, the injury led to a progressive increase in the left ventricular (LV) end-diastolic and end-systolic volumes, a reduction in the LV ejection fraction (LVEF), an increase in the sphericity index and hypertrophy of myocytes. At 8 weeks after injury, the sheep that developed chronic dilated cardiomyopathy with LVEF of <40% were included in the reverse remodelling study. Sheep that met the inclusion criteria were randomised to either Impella 5.5 support with maximum unloading (n=7) or a control group without Impella support (n=7). The sheep were supported for 3 months and sacrificed at the 5-month time point for tissue collection. Assessments, including cardiac MRI, haemodynamic measurements, biopsies and blood analysis, were taken prior to injury (baseline), at the point of randomisation (8 weeks) and at the end of the study (5 months). The study endpoints are listed in Table 1.
In his talk, Dr Meyns presented the results of the functional assessment. Structural changes were demonstrated via cardiac MRI from 8 weeks to 5 months. Although the magnitudes of impact varied, sheep who were supported by the Impella 5.5 showed an overall improvement in the end-diastolic and end-systolic volumes, with those that had the most dilated LV chambers showing the most reverse remodelling. Although there were significant reductions in the end-diastolic and end-systolic volumes in the Impella-supported group, there were no significant group effects on the overall stroke volume, sphericity index, cardiac output or LVEF.
In this model of chronic ischaemic cardiomyopathy, scar size did not change from 8 weeks to 5 months. Interestingly, regional analysis of the remote zone by MRI showed a significant improvement in function in the Impella-supported group: regional LVEF, wall thickening and wall volumes were reduced in the remote zone. This finding was confirmed by histology results, which showed a significant reduction in the mean number of myocytes in the remote zone. Dr Meyns clarified that further analyses on the functional and molecular aspects are pending, including analysis of plasma samples, biopsies, pressure–volume loops and Impella pressure sensor data.
The data shown by Dr Meyns demonstrate that long-term unloading with Impella achieves reverse remodelling of the chronic failing heart with a significant reduction in the global end-diastolic and end-systolic volumes and cardiac hypertrophy. In addition, Impella support improved regional contractility in the remote zone. These findings offer hope for patients with chronic heart failure. Dr Meyns’ team is working on the molecular analysis of the tissue samples collected from this study to add to the current functional analysis.