Coronary CT angiography (CTA) is a well-established tool for diagnosing coronary artery disease (CAD). Coronary CTA also facilitates the assessment of a patient’s risk based on plaque characteristics.1 Current guidelines recommend using coronary CTA as the initial assessment for patients with chest pain.2 Additionally, fractional flow reserve (FFR) can be estimated with conventional coronary CTA, providing a combined anatomical and functional evaluation in one test. FFR derived from CT (FFRCT) technology also enables virtual percutaneous coronary intervention (PCI) and predicts the degree of functional revascularisation following PCI using the FFRCT Planner.3 Studies and meta-analyses have linked the degree of functional revascularisation after PCI, measured by post-PCI FFR, to hard outcomes.4 This level of outcome prediction is unprecedented and could impact the CAD field, leading to improved patient outcomes. This review focuses on describing the basics and perspectives of coronary CTA analysis, emphasising the role of artificial intelligence in the planning and guidance of revascularisation procedures.
Risk Assessment Based on Coronary CT Angiography Before Revascularisation
Prior to considering revascularisation, coronary CTA effectively stratifies patients in terms of procedural outcomes. Procedural risk associated with PCI can be estimated based on disease complexity and the anatomical features of the lesion to be treated. Coronary CTA allows risk stratification according to disease extension (e.g. one-vessel disease, two-vessel disease, three-vessel disease or left main disease).5 Anatomically high-risk procedures can be defined by the type of lesion, disease affecting major bifurcations, calcification severity or the presence of total coronary occlusions. Moreover, lipidic plaque composition based on coronary CTA is associated with periprocedural injury, probably because of material embolisation to the coronary microcirculation. Peri-procedural MI has been shown to be higher in lesions with high-risk plaque characteristics.6 Lipid-rich plaque with the napkin ring sign and low-attenuation plaque has been linked to risk of a no-reflow during PCI.7–9 These high-risk plaque characteristics, e.g. positive remodelling and low-attenuation plaque, also carry a higher risk for spontaneous MI. To correctly identify these plaques, algorithms for vessel wall detection are being enriched by deep learning methods to provide measurements of plaque volume and composition that closely correlate with intravascular imaging.10 Artificial intelligence algorithms are increasingly being applied to coronary CTA to improve the efficiency, reproducibility and accuracy of image analysis.11 These algorithms have proven to have an excellent capacity for predicting MI in patients with CAD. The next step is to apply these methods to pre-procedural stratification in patients considered for revascularisation. The availability of coronary CTA for stratification based on artificial intelligence may inform the best treatment strategy. Therefore, the risk of an intervention based on patient-specific information could be established, enhancing the decision-making process about revascularisation and allowing procedural planning.
Coronary Physiology Derived From Coronary CT Angiography
FFRCT is calculated by applying computational fluid dynamics (CFD) to coronary geometries extracted from CTA. FFRCT is then defined as the computed mean coronary pressure distal to a lesion divided by the mean pressure in the aorta under simulated maximal hyperaemia.3 The FFRCT results are presented on a 3D model of the coronary arteries, and colour-coded based on the FFRCT value at each level. Nørgaardet et al. demonstrated that the use of FFRCT, compared with a visual assessment alone, improved the diagnostic performance of the detection of haemodynamically significant lesions.12 Furthermore, FFRCT facilitates the assessment of the functional pattern of CAD, i.e. focal or diffuse, by providing longitudinal vessel information mimicking invasive pressure pullbacks.13 Identifying the functional CAD pattern beyond the distal FFR value alone not only influences the revascularisation strategy but also provides additional risk stratification for plaque rupture and MI.14 Moreover, the trans-lesional pressure gradient of FFRCT (ΔFFRCT) is an independent predictor of MI. Furthermore, the haemodynamic patterns of CAD are linked to the benefit of PCI in terms of angina relief. PCI restores epicardial conductance in cases of focal CAD, whereas blood flow improvement after PCI in cases of diffuse disease is significantly lower.15 The pattern of CAD, quantified by the pullback pressure gradient (PPG), has been associated with freedom from angina after PCI; residual angina is almost twice as common in patients with a low PPG (haemodynamic diffuse disease) compared with high PPG (focal disease).16 Thus, the addition of functional information derived from CTA enhances risk prediction at the lesion level while providing information on the clinical benefit of revascularisation in terms of angina relief. The FFRCT technology leverages machine-learning-based techniques to segment the coronary geometry that is used for CFD as well as to segment plaque volumes and types. Moreover, machine-learning techniques can be applied to improve the accuracy of the FFR predictions using invasive data or by replacing the necessity of CFD. From a clinical perspective, the addition of reliable physiological information to coronary CTA improves cath lab efficiency by providing a complete physiological map without the need for a pressure wire.17 Two case examples of the application of the planner in cases of focal and diffuse CAD are shown in Figure 1.
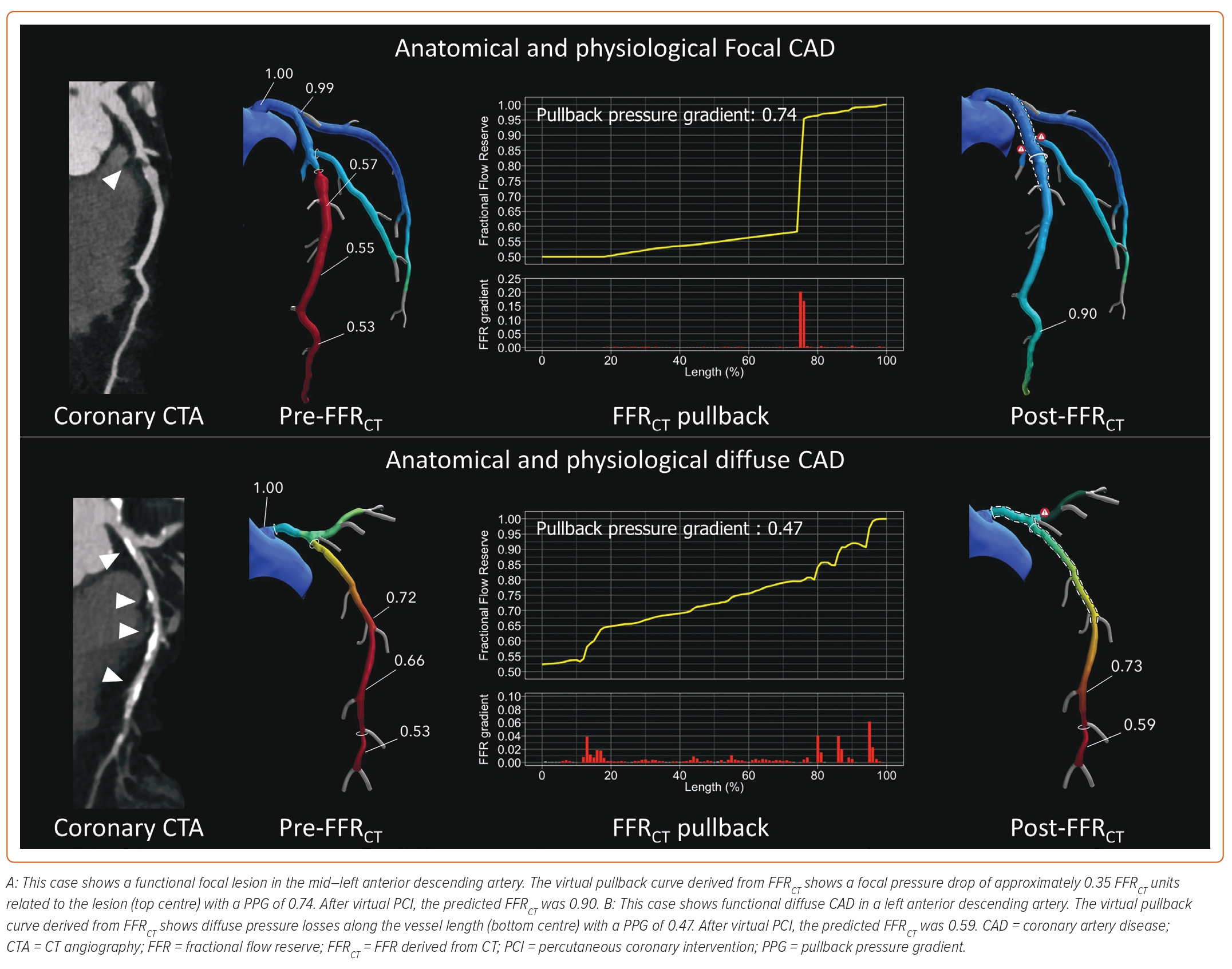
The FFRCT Planner (HeartFlow) is a novel tool that predicts FFR in response to changes to patient-specific lumen geometry in real time. The FFRCT Planner recalculates FFR after the virtual removal of coronary artery stenoses predicting post-PCI FFR. Post-PCI FFR is a metric of the degree of functional revascularisation and has been identified as an independent predictor of cardiac death and MI after PCI.4 Sonck et al. prospectively validated this tool showing high accuracy and precision with invasively measured post-PCI FFR as a reference.18 In addition, in cases of tandem lesions, FFRCT is the most accurate tool to predict the interactions or cross-talk between lesions, outperforming even invasively derived algorithms. As with the FFRCT technology, the FFRCT Planner is powered by CFD. Several pre-computations are performed to understand the relationship between flow and resistance in every vessel location. Planned lumen changes modify the expected flow rate through the vessels and are used with the geometry change to calculate a new FFRCT along the vessel.19
Myocardial Mass at Risk
Vessel-specific myocardial mass can be quantified from the coronary CTA images using either the Voronoi’s algorithm or CFD-based technique. These software programmes automatically provide the territory-specific myocardial mass.3,20–22 The information provided by the mass is used during the CFD simulations to estimate the amount of blood flow going through each of the coronary vessels. The estimation of blood flow is essential information to accurately calculate trans-lesional pressure gradients. In addition, the subtended myocardial mass has been associated with FFR and myocardial ischaemia.23,24 For PCI planning, myocardial mass information is of particular interest in the case of bifurcation lesions, when knowing the mass at risk of the side branch may help determine the PCI bifurcation strategy in terms of side branch protection or stenting. It should be underlined that the current process of mass analysis does not distinguish between viable and non-viable myocardium. Nonetheless, tissue characterisation by CT with the combination of CT perfusion allows a scar to be distinguished from healthy myocardium.25
Cath Lab Setup Based on Coronary CT Angiography
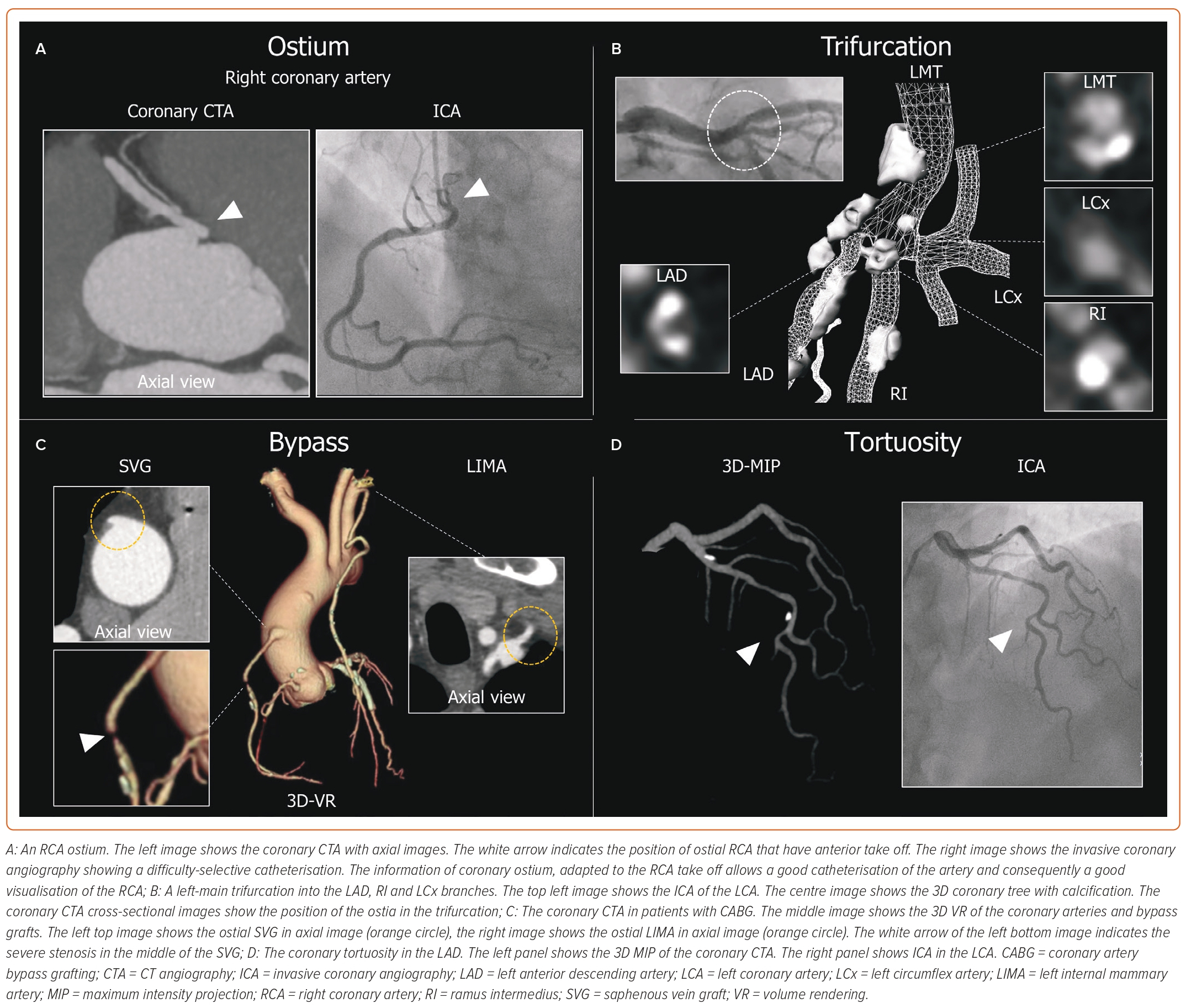
The anatomical information on coronary anatomy and function obtained by coronary CTA helps to plan the procedure by selecting and preparing the material upfront and defining the PCI strategy. For example, adequate catheter support is critical for PCI, especially in complex disease. The evaluation of the coronary artery take-offs in the axial views from CTA assists in selecting the most appropriate guiding catheter. An anterior take-off of the ostial right coronary artery results in insufficient support from classical catheters (e.g. Judkins right) and makes one consider selecting an Amplatz left guiding catheter. Thus, by anticipating the most appropriate catheter, coronary CTA allows operators to save resources, contrast and procedural time, while avoiding catheter exchanges during the procedure. In addition, in other types of coronary anomalies and in bypass grafts, coronary CTA is the preferred imaging modality to locate the ostium of the vessels. Further studies are necessary to understand how to select the most appropriate catheter for each anatomy where cannulation simulations might be useful. Coronary CTA is useful for patients previously treated by coronary artery bypass grafting (CABG) and planned to undergo coronary angiography. The presence of CABG creates per se a complex scenario, as catheterisation of the bypass grafts can be challenging and time-consuming. Coronary CTA can define the number, ostium location, target native coronary and patency of the bypass grafts. Potential lesions on the bypass graft can also be visualised.26 These findings are not only useful for material selection but may also impact arterial access. Recently, the BYPASS-CTCA study demonstrated that, in patients who had previously undergone CABG and were referred for an invasive coronary angiography, coronary CTA planning increased the use of radial access along with reducing procedural duration and complications while improving patients’ satisfaction.27 The case examples of beneficial information on the coronary anatomy obtained by coronary CTA are shown in Figure 2.
Coronary CTA can provide data on the entry cap (blunted or tapered), calcium severity, the presence of calcium at the entry and exit of the chronic total occlusion (CTO) and the presence of multiple occlusions, among other features. Clinical trials addressing the value of CT for CTO planning have shown that CT contributes to a higher success rate compared with no CT planning.28,29 There is the potential for artificial intelligence algorithms to – in addition to detecting stenosis and identifying plaque composition – also provide information about PCI planning.
Coronary Calcification
One of the main limitations of coronary CTA for diagnostic evaluation is the presence of calcium, which hampers luminal evaluation and frequently leads to an overestimation of disease severity. For PCI planning, the high sensitivity of CT for calcium represents a unique opportunity to determine the severity of calcifications. Patients with severe calcifications undergoing PCI have a significantly higher rate of target-vessel failure because of higher procedural risks and reduced stent durability.30 Coronary CTA can provide a precise evaluation of the calcium burden, arc extension, thickness, length and distribution.31 However, it is relevant to mention that – at this stage – coronary CTA is unable to assess the location of calcium (superficial versus deep) and to differentiate nodular from other types of calcifications. Nonetheless, providing an accurate assessment of calcium arc and burden coronary CTA informs the necessity of plaque-modification techniques such as a cutting balloon, intravascular lithotripsy, rotational atherectomy (RA) and orbital atherectomy during the PCI. Recent observational reports have demonstrated an association between calcium characteristics and the need for RA.32,33 Sekimoto et al. showed that calcium score and arc are associated with the need for RA during PCI, and recently Kurogi et al. reported that the mean density of the cross-sectional CT image, potentially representing information about calcific strength, is the strongest predictor of the need for RA during PCI.32,33 Therefore, the application of artificial intelligence for the accurate segmentation of calcific plaques, with the potential for a more detailed stratification of calcific plaque, is a promising field. In the future, coronary CTA-based algorithms will likely be used to determine the necessity of calcific plaque modification with dedicated devices.34
Online Percutaneous Coronary Intervention Guidance
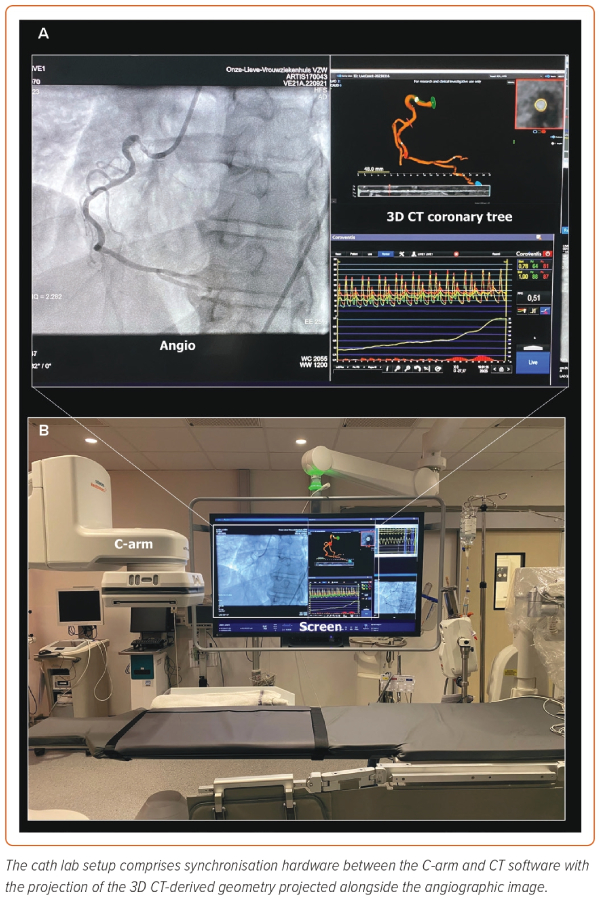
Coronary CTA is now being tested as a tool to guide PCI; this approach provides the possibility of assessing plaque extension and distribution during invasive coronary angiography. The coronary tree with 3D reconstructions of the plaque in the three vessels is projected onto the screen in the cath lab during the procedure, alongside the invasive coronary angiography (QAngioCT Cath Lab, Medis Medical Imaging Systems; Figure 3). The 3D coronary tree model is synchronised in real-time to the C-arm position to match the views with the fluoroscopic arm and continuously visualised following changes in angiographic projections (Supplementary Video 1). This approach enables finding the optimal view without contrast injection or radiation. Moreover, it facilitates the interpretation and distinction of plaque components, as they are colour-coded according to their Hounsfield units.
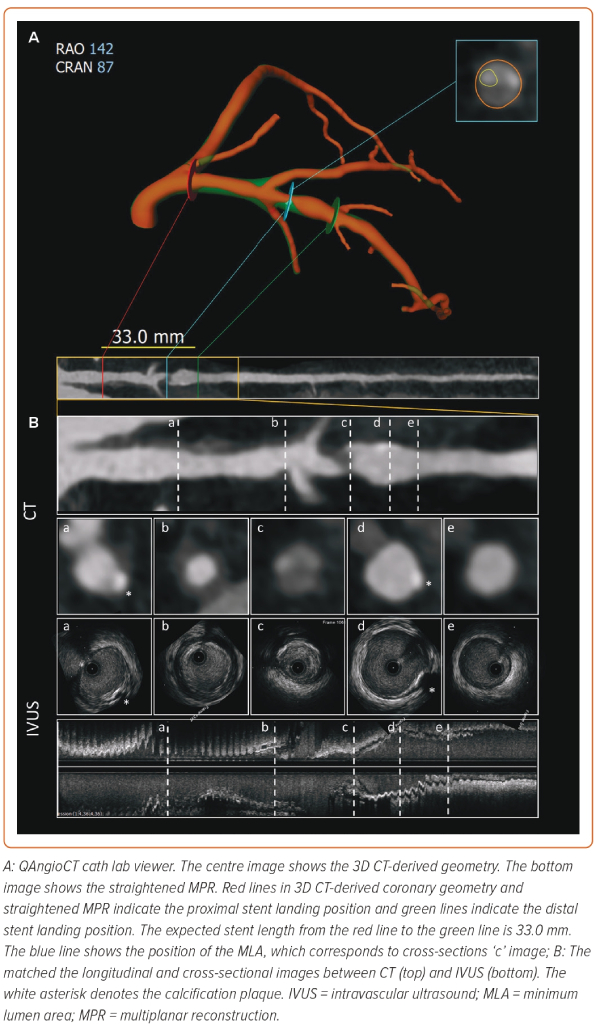
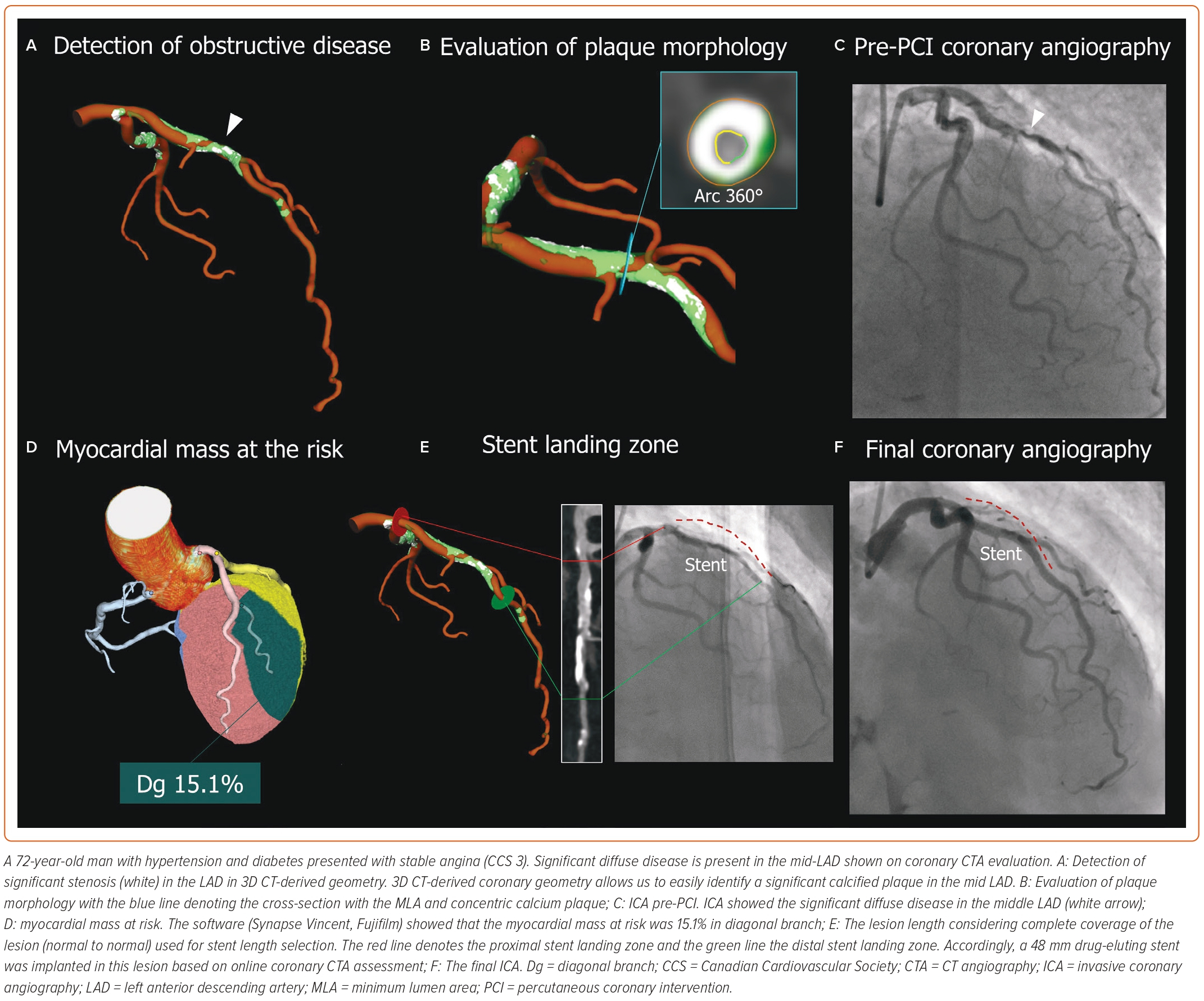
PCI guidance using coronary CTA starts with the identification of significant obstructive disease and the evaluation of plaque characteristics and disease extension. The additional benefit of coronary CTA is that it provides a 3D depiction of the complete tree with atherosclerotic plaque characterisation, similar to intravascular imaging (Figure 4). Thus, by simultaneously displaying the plaque in 3D, the coronary CTA overcomes one of the main drawbacks of the angiogram, which shows only the lumen. This additional information provides a complete depiction of the disease and plaque types and assists in stent deployment.35 Moreover, it allows for real-time measurements to adjust the PCI strategy. While the current approach relies on semi-automatic segmentation of the coronary tree, future iteration based on artificial intelligence will allow for rapid reconstruction and plaque characterisation with treatment recommendations based on the patient’s specific disease. A case example of CT-guided PCI is shown in Figure 5.
Conclusion
Leveraging artificial intelligence, the reconstruction process will be fully automated, providing reproducible and comprehensive coronary CTA assessments for PCI planning that will be likely to influence outcomes, resulting in a cost-saving approach.36 Deep learning using large amounts of data has the potential to improve speed, diagnostic performance and image analysis of coronary CTA by providing automated and objective results. The integration of coronary CTA inside the cath lab is a novel approach to CAD diagnosis and treatment. CT-guided PCI has the potential to increase the use of imaging-guided PCI. The ongoing P4 clinical trial (NCT05253677) is currently investigating whether a CT-guided PCI approach results in similar outcomes compared with intravascular ultrasound-guided PCI. Coronary CTA could likely become the predominant imaging modality for PCI guidance.