Case Presentation
A 65-year-old man presented with limiting Canadian Cardiovascular Society class III stable angina symptoms despite optimal medical therapy. This followed a recent admission to hospital with an unstable angina presentation, which was managed medically.
His medical history included hypertension, hyperlipidaemia and gout. In addition, he had established coronary artery disease; 14 years prior, he had undergone coronary artery bypass grafting (CABG) with a left internal mammary artery (LIMA) to the left anterior descending (LAD) artery with triple jump radial artery conduits to the first diagonal with a side to side anastomosis, first obtuse marginal (OM1) with an end to side anastomosis and right posterior descending artery (RPDA) with an end to side anastomosis. A dobutamine stress echocardiogram revealed inducible ischaemia in the inferior and lateral left ventricular walls, so the patient was subsequently listed for a diagnostic coronary angiogram.
Procedural Details
As the left radial had been harvested and the left ulnar pulse was weak, the procedure was carried out via the right femoral artery initially with a 6 Fr sheath (Cordis). Diagnostic coronary angiography revealed a severe stenosis at the radial artery graft bifurcation site between the continuous radial artery from the LIMA running to the RPDA and the jump radial segment to the OM1. The native right coronary artery (RCA) was a dominant vessel and had a chronic total occlusion (CTO) in the mid vessel with minimal antegrade flow and very little retrograde flow to the distal RCA from the left coronary artery. The LAD was occluded after a large first septal artery and the circumflex (Cx) was a small vessel with a patent distal atrioventricular Cx but occluded obtuse marginal branches (Figure 1).
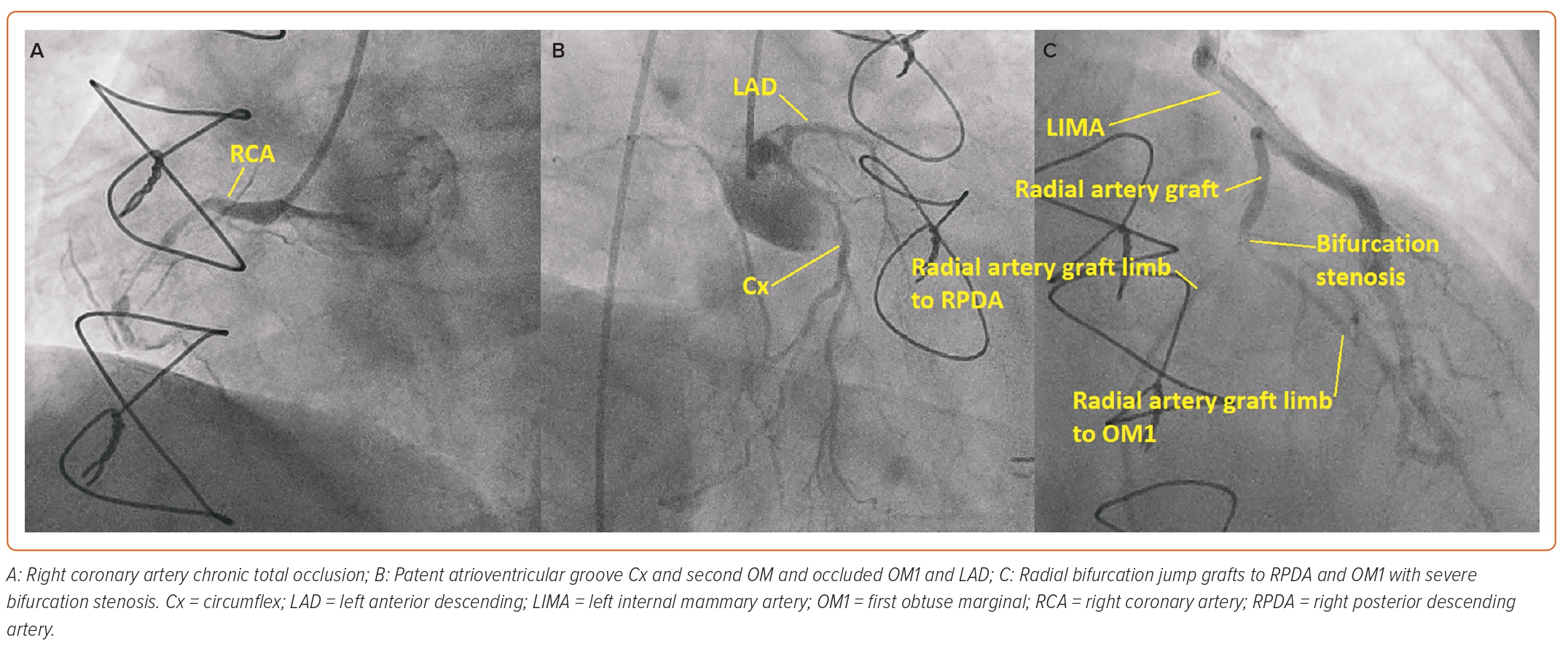
It was felt that percutaneous coronary intervention (PCI) to the native RCA CTO would provide the patient with the best symptomatic relief. As a temporary measure before bringing the patient back for a dual access CTO PCI at a later date when better set up to perform it, it was decided to treat the radial artery graft bifurcation disease with drug-coated balloons at that sitting.
The femoral sheath was upsized to a 7 Fr sheath (Cordis), a 90 cm 7 Fr internal mammary guide catheter (Medtronic) was used to engage the LIMA and two Sion blue wires (Asahi Intecc) were placed down each radial artery limb beyond the bifurcation disease. Each limb was pre-dilated with a 2.5 × 12 mm semi-compliant balloon (Boston Scientific). Passage of a 2.5 × 15 mm SeQuent paclitaxel drug-coated balloon (B Braun) was challenging because of the LIMA tortuosity.
Drug-coated balloon deployment of the RPDA segment of the radial artery graft did not achieve a ‘stent-like result’, with significant recoil noted, so crossover to placement of a stent was decided upon. Again, passage of a 2.5 × 15 mm Onyx drug-eluting stent (DES; Medtronic) was difficult, but eventually the stent was passed into the RPDA portion of the radial graft across the bifurcation with the OM1 jump and deployed at this site.
Soon after deployment of this stent, the patient developed chest pain and shortly afterwards sustained VF. He received three stacked shocks at 360 J and 2 minutes of cardiopulmonary resuscitation before return of spontaneous circulation was achieved. Tentative injection via the internal mammary guide catheter with the catheter disengaged into the left subclavian artery revealed iatrogenic dissection of the LIMA with no antegrade flow. The most likely mechanism of dissection was over intubation of the guide catheter during difficult manipulation of the stent through tortuosity, causing catheter-induced dissection.
At this juncture, the cardiac surgical team were consulted and were on standby to undertake emergency surgery if the situation could not be rectified percutaneously. The inlet of the suspected dissection site, i.e. the ostium of the LIMA, was secured by placing a 4 × 12 mm Onyx DES but despite this, antegrade flow was not achieved, indicating that the dissection had propagated further into the LIMA beyond the distal edge of the stent (Figure 2).
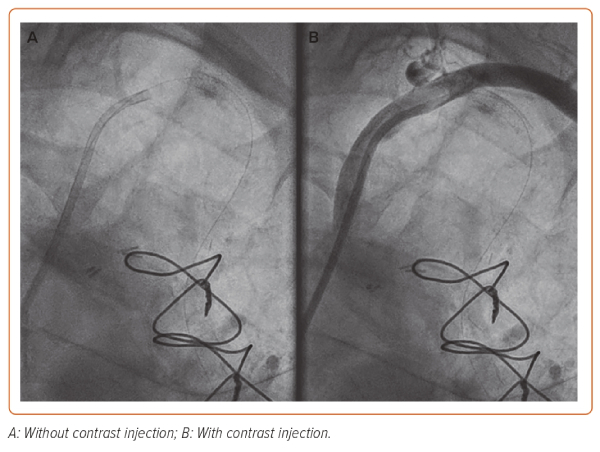
Unfortunately, intravascular ultrasound (IVUS) was not available as the portable console was being used in another cath lab for an emergency case. A more distal DES was placed (4 × 12 mm Onyx DES) in what was thought to be beyond the dissection in healthy vessel. To confirm placement of the distal stent and sealing of the outlet, an Export AP thrombus aspiration catheter (Medtronic) was passed into the LIMA and positioned at the distal end of the LIMA. Contrast was then injected via the aspiration port while pulling back slowly on the catheter (Supplementary Video 1). This technique limits the propagation of dissection from hydraulic forces from standard injection via the guide catheter but allows adequate visualisation of the vessel to assess the level of the dissection while also maintaining wire position in the distal vessel.
A similar procedure can be performed with a dual-lumen microcatheter, which has both rapid exchange and over-the-wire exit ports of which the over-the-wire hub allows the injection of contrast.1,2 Such catheters include the Twin-Pass and Twin-Pass Torque (Teleflex), Sasuke (Asahi Intecc) and NHancer RX (IMDS) microcatheters. In emergent situations where neither IVUS nor dual-lumen catheters or their equivalent are available, standard semi-compliant balloons can be inflated ex-vivo using saline then numerous perforations made in the balloon with a micro puncture needle. The balloon is then introduced into the vessel segment of interest and contrast injected via the balloon hub. While this technique has largely been described for the delivery of pharmacological agents in the context of no reflow, the author has also found it helpful as means of localised contrast delivery.3,4
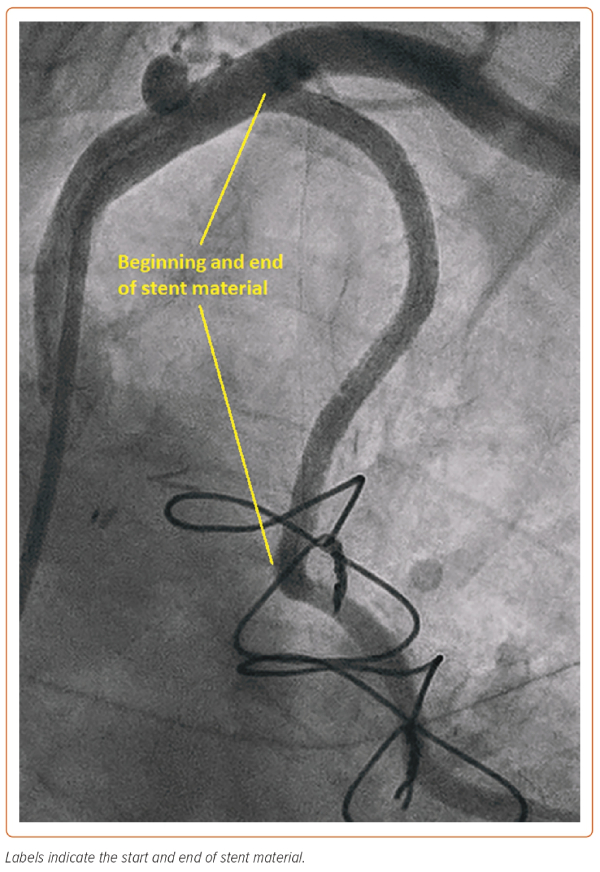
In this case, the manoeuvre using the Export thrombus aspiration catheter demonstrated that the more distal stent had sealed the dissection outlet. Such stenting beyond the dissection into normal vessel avoids so called ‘tooth-pasting’, i.e. propagation of the dissection downstream. The gap in between the inlet and outlet stents was filled to fully seal the dissection and, in total, four DES stents were placed. The stents were post-dilated with a 4 mm non-compliant balloon (Boston Scientific) to 16 atm. A standard injection through the guide catheter then revealed restoration of brisk flow and well-sized stents, with the stent material covering the LIMA ostium and extending around 1 mm back into the left subclavian artery (Figure 3). It was noted that there was loss of flow into the radial jump supplying the OM1 graft, but as the patient was pain free and stable at this point, it was decided to manage this conservatively (Figure 4).
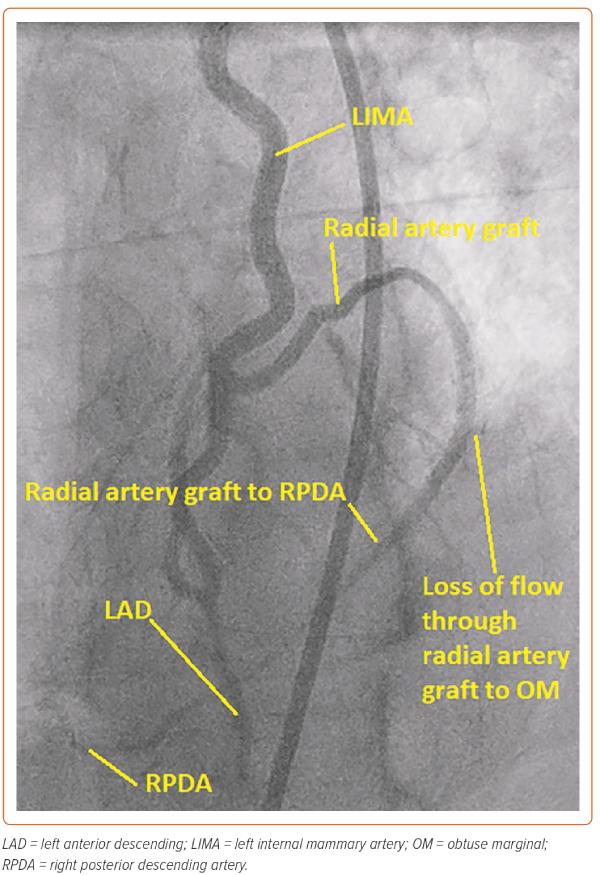
At follow-up, the patient reported a significant improvement in his angina and he declined CTO PCI of the RCA.
Discussion
The use of the radial artery as a graft conduit has become popular with cardiac surgeons because of its favourable rates of occlusion compared with saphenous vein grafts and its long length lending itself to multiple sequential anastomosis.5,6 Despite the superiority of radial grafts over saphenous vein grafts in terms of long-term patency rates in the modern surgical era, degeneration of radial grafts can still occur.
In a case series of radial graft PCI, Sharma et al. showed that approximately two-thirds of cases of radial artery graft stenosis occurred at the anastomosis site, as was seen in this case, i.e. disease between the anastomosis of the shorter radial artery segment to the obtuse marginal onto the longer radial artery graft segment to the RPDA.7,8 They described favourable outcomes of radial graft PCI in this small series. These findings were echoed in more recent case series included in a large study by Hamilton et al. with comparable 3-year mortality data to native-vessel PCI.9
Ideally, complex cases such as this should go through a Heart Team and have an individualised assessment of the risk for PCI versus redo-CABG. An emerging risk calculator in the UK for assessing PCI risk in complex high-risk indicated PCI is the British Cardiovascular Interventional Society risk calculator.10 Of note, in this risk calculator, graft PCI is not one of the procedural factors associated with increased major adverse cardiac or cerebrovascular events.
While outcomes of radial artery graft PCI appear favourable, in many circumstances the grafts are anastomosed via the LIMA and not directly to the aorta. In such cases, significant care must be taken to avoid catheter-induced iatrogenic coronary artery dissection of the LIMA. The case reported here was especially significant as the dissection led to complete loss of antegrade flow, i.e. a National Heart, Lung, and Blood Institute (NHLBI) Type F dissection. This led to profound haemodynamic compromise and cardiac arrest, as the LIMA supplied the majority of the myocardium.11 Recent data from a large registry from the NHLBI of 55,968 patients undergoing coronary angiography over a 10-year period found a prevalence of catheter-induced ostial coronary artery dissection of 0.09%, of which 66% were Type F dissections. The majority of these cases were treated with PCI.12
As well as the traditional method of sealing the inlet and outlet of a dissection, a number of other techniques have been described to manage iatrogenic catheter-induced coronary dissections, including the use of a cutting balloon to decompress intramural haematoma or aspiration of blood from the subintimal space akin to the subintimal transcatheter withdrawal technique used in CTO PCI antegrade dissection re-entry (ADR) crossing.13,14 If the wire position is lost, other CTO techniques can be used to regain distal luminal wire position such as retrograde crossing through collaterals or ADR.15
Of course, prevention is better than cure, and to mitigate against the risk of catheter-induced dissection of the LIMA during PCI, a number of authors have extolled the benefits of the use of mother and child guide extension catheters, such as the Guideliner (Teleflex), Guidezilla (Boston Scientific) or Telescope (Medtronic).16–18 These devices prove especially beneficial when coaxial alignment of the guide catheter with the LIMA ostium cannot be achieved. Care must be taking when advancing guide extension catheters deep into tortuous and/or diseased LIMAs and it is recommended to use a balloon to soften the tip and use the inch worming technique if necessary to avoid LIMA dissection.19
Conclusion
Given the LIMA graft’s long-term excellent patency rates and relative protection against atherosclerosis, it is to be revered.20 In the author’s opinion, PCI that uses the LIMA as a conduit should be reserved for scenarios where other viable options have been exhausted and the full risks of the procedure are understood by both the interventional cardiologist and the patient. In this report, we present a case of guide catheter-induced LIMA dissection during an attempt to treat radial artery jump graft bifurcation disease leading to haemodynamic compromise. In the absence of IVUS availability, the use of contrast injection through a thrombus aspiration catheter allowed for confirmation that the distal edge of the dissection had been sealed by a DES and safe recovery of the LIMA. 









