Coronary artery disease (CAD) is associated with the risk of atherothrombotic events and warrants long-term antithrombotic therapy. The standard treatment for CAD patients who present with acute coronary syndrome (ACS) or chronic coronary syndrome (CCS) includes single (SAPT) or dual antiplatelet therapy (DAPT), sometimes in combination with oral anticoagulants (OACs).1,2 However, establishing the appropriate composition and duration of this standard treatment poses challenges in view of the associated risk of bleeding. Therefore, it is important to tailor the treatment according to individual ischaemic and bleeding risks.
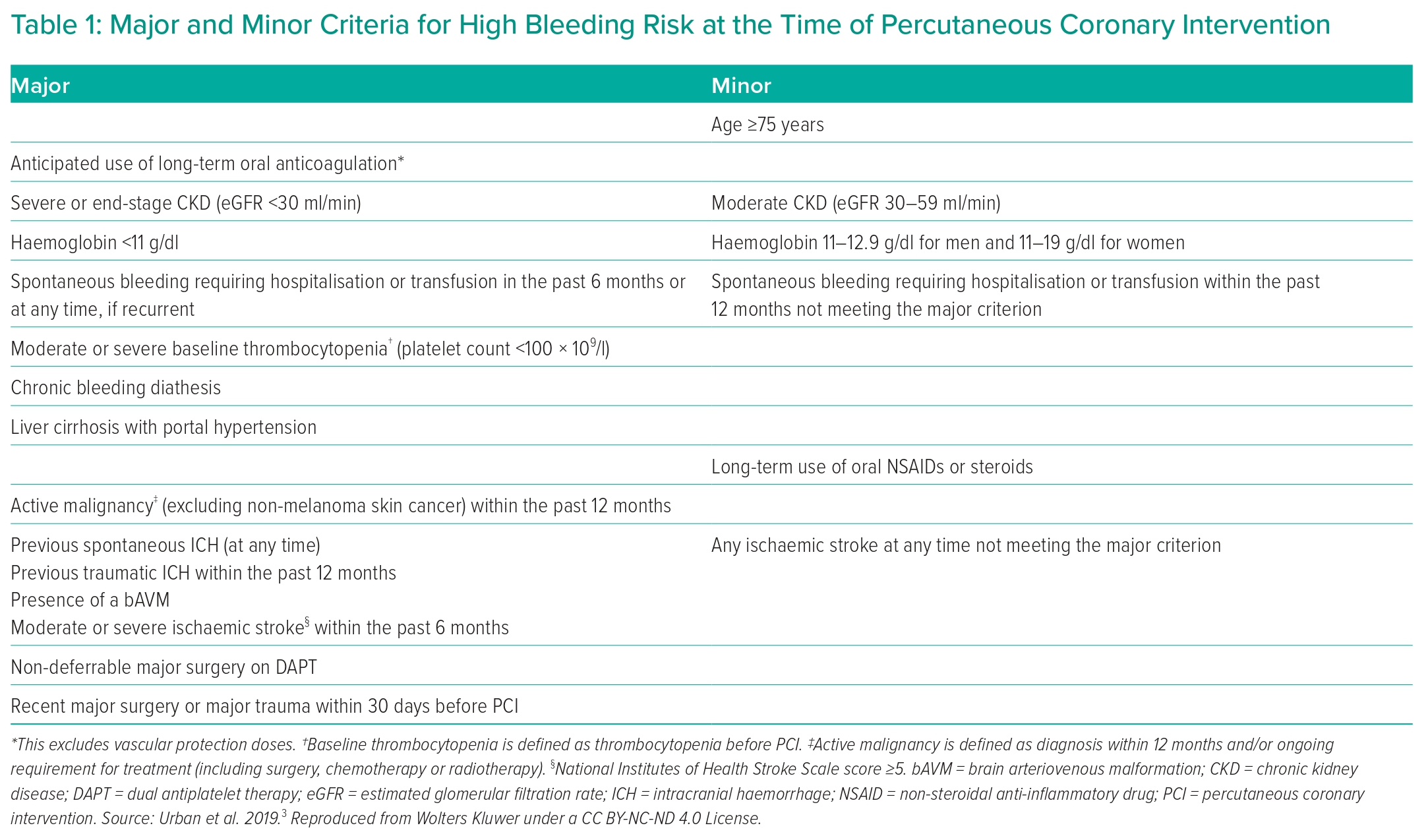
European Society of Cardiology (ESC) guidelines have been revised to identify patients who would benefit from either shortened or extended DAPT, or potent or less potent antithrombotic therapy.1,2 They recommend evaluation of both ischaemic and bleeding risk using a number of risk scores and tests that are available to facilitate this, while acknowledging that further work is required to explore any impact of the use of bleeding risk scores on clinical outcomes. Empirically, determining the optimal treatment is dependent upon assessing a comprehensive range of haemorrhagic and ischaemic risk factors while considering each patient’s clinical characteristics. The Academic Research Consortium (ARC) has identified major and minor bleeding risk factors that can identify patients with high bleeding risk (HBR; Table 1).3
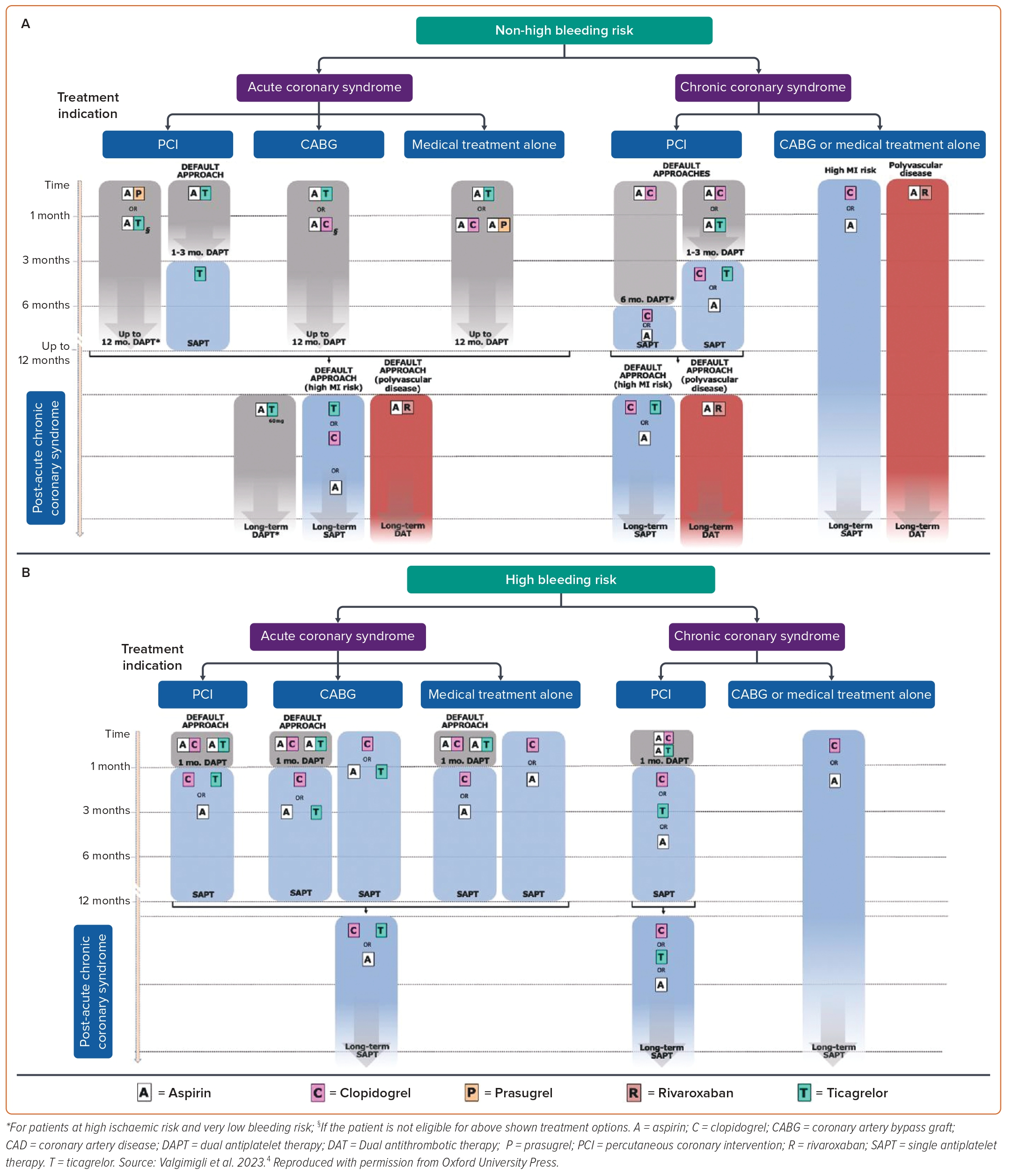
We use four case studies to highlight the considerations that must be made in deciding on the optimum antithrombotic strategy, based on the available guidelines. Given the choices available according to the ESC guideline recommendations, we also draw on the strategies highlighted in a recent European expert consensus statement, which used published network meta-analyses to rank competing antithrombotic strategies.4 In line with these, we support a tailored antithrombotic approach after assessment of individual risk factors.
Case Study 1: ACS with High Ischaemic Risk and without High Bleeding Risk
A male patient, aged 63 years, presented following a 1-hour episode of central chest pain. He had a history of hypertension and type 2 diabetes. He was a current smoker and was taking a statin, a β-blocker and an oral antihyperglycaemic. He had stage 3 chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 at admission. Haemoglobin was normal at 146 g/l with normal mean corpuscular volume (MCV). ECG showed an absence of ST segment elevation. High-sensitivity troponin was initially mildly elevated and 1 hour later was above the threshold for rule-in of non-ST-elevation MI (NSTEMI). Coronary angiography was scheduled for the following day, subject to cath lab availability. This was performed 28 hours after presentation and showed diffuse mild disease of the dominant right coronary artery (RCA) with a 50% stenosis at the origin of the posterior descending artery (PDA), mild left main stem disease and a severe bifurcation stenosis (1,1,1) of the proximal left anterior descending (LAD) artery and first diagonal branch. He underwent percutaneous coronary intervention (PCI) with upfront two-stent strategy using the mini-crush technique of both the diagonal artery (2.5 mm × 15 mm everolimus-eluting stent [EES] with 2.75 mm × 10 mm non-compliant [NC] balloon) and the LAD artery (3.0 mm × 20 mm EES to mid-LAD and overlapping 3.5 mm × 24 mm EES postdilated with 3.5 mm× 12 mm NC balloon). Imaging confirmed achievement of good stent deployment.
Treatment of This Patient Based on Current Guidance
This patient was treated with a loading dose (LD) of aspirin 300 mg. Given that coronary angiography could not be performed on the same day and NSTEMI was diagnosed, he was treated with an LD of ticagrelor 180 mg followed by 90 mg twice daily. It was uncertain whether coronary angiography would be performed within 24 hours and therefore subcutaneous fondaparinux 2.5 mg was administered. Unfractionated heparin (UFH) 70 U/kg was administered at the time of PCI. After undergoing PCI, the patient’s long-term ischaemic risk was considered high in view of multivessel CAD, type 2 diabetes and stage 3 CKD. His bleeding risk was not considered to be high due to the absence of any major ARC HBR characteristic and the presence of only one minor ARC HBR characteristic, namely CKD with eGFR in the range 30–59 ml/min/1.73 m2 (two minor or one major ARC HBR criteria are required to indicate HBR). It was planned to continue low-dose aspirin and ticagrelor 90 mg twice daily for 12 months followed by aspirin and ticagrelor 60 mg twice daily in the long term thereafter, if tolerated, in view of the high ischaemic risk with multiple ischaemic risk factors combined with the lack of HBR. This decision was to be reviewed if he developed any HBR conditions.
This management is consistent with the ESC 2023 ACS guidelines, which have the following recommendations:2
- Aspirin is recommended for all patients without contraindications at an initial oral LD of 150–300 mg (or 75–250 mg IV) and a maintenance dose (MD) of 75–100 mg once daily for long-term treatment. Class 1, level of evidence a.
- In all ACS patients, a P2Y12 receptor inhibitor is recommended in addition to aspirin, given as an initial oral LD followed by an MD for 12 months unless there is HBR. Class 1, level of evidence a.
- Ticagrelor is recommended irrespective of the treatment strategy (invasive or conservative) (180 mg LD, 90 mg twice-daily MD). Class 1, level of evidence b.
- Pre-treatment with a P2Y12 receptor inhibitor may be considered in non-ST-segment elevation acute coronary syndrome (NSTE-ACS ) patients who are not expected to undergo an early invasive strategy (<24 hours) and do not have HBR. Class 2b, level of evidence c.
- For patients with NSTE-ACS in whom early invasive angiography (i.e. within 24 hours) is not anticipated, fondaparinux is recommended. Class 1, level of evidence b.
- Routine use of a UFH bolus (weight-adjusted IV bolus during PCI of 70–100 IU/kg) is recommended in patients undergoing PCI. Class 1, level of evidence c.
- Adding a second antithrombotic agent to aspirin for extended long-term secondary prevention should be considered in patients with high ischaemic risk and without HBR. Class 2a, level of evidence a.
The strategy of continuing long-term DAPT is also consistent with the European expert consensus document that recommends long-term DAPT with aspirin and ticagrelor 60 mg twice daily in those with high ischaemic risk and ‘very low bleeding risk’.4 However, the proportion of ACS patients with this risk combination is small. An alternative strategy for long-term dual antithrombotic therapy is the combination of aspirin with rivaroxaban 2.5 mg twice daily.
Influence of Timing of Coronary Angiography on Guideline Recommendations
If coronary angiography had been performed on the same day as admission, then the 2023 ESC guidelines recommend against treatment with an oral P2Y12 inhibitor before angiography and, under these circumstances, recommend that prasugrel should be considered in preference to ticagrelor in ACS patients who proceed to PCI (class 2a, level of evidence b), based predominantly on the ISAR-REACT 5 study results.2 The evidence for and against these recommendations has been reviewed in a previous debate.5 The guidelines also state that ‘parenteral anticoagulation is recommended for all patients with ACS at the time of diagnosis’ and recommend that ‘for patients with NSTE-ACS in whom early invasive angiography (i.e. within 24 h) is anticipated, enoxaparin should be considered as an alternative to UFH’ and ‘Intravenous enoxaparin at the time of PCI should be considered in patients pre-treated with subcutaneous enoxaparin’. This parenteral anticoagulation provides a bridge to coronary angiography when oral P2Y12 inhibition is withheld in NSTEMI patients. Consequently, it is implied by the ESC guidelines that the parenteral anticoagulation strategy should be determined by the planned timing for PCI, with subcutaneous enoxaparin or IV UFH given in those planned for invasive angiography within 24 hours, whereas subcutaneous fondaparinux is favoured when invasive angiography will be delayed >24 hours, under which circumstances pre-treatment with an oral P2Y12 inhibitor may also be considered.
Case Study 2: Acute Coronary Syndrome with High Bleeding Risk
A male patient, aged 68 years, presented following a 1-week history of new-onset exertional angina culminating in a 30-minute episode of central chest pain at rest. He had a history of hypertension, hyperlipidaemia and minor ischaemic stroke 3 years earlier with no significant residual disability. He was a non-smoker and was taking ramipril, amlodipine, clopidogrel and a moderate-intensity statin regimen. He had stage 3 CKD with eGFR of 50 ml/min/1.73 m2 at admission. Haemoglobin was below the lower limit of normal for men at 124 g/l with normal MCV. ECG showed T wave inversion in the inferior leads and an absence of ST segment elevation. His high-sensitivity troponin at admission was above the threshold for rule-in of NSTEMI. Coronary angiography was scheduled for the following day. This was performed 20 hours after presentation and showed normal left main stem, mild stenoses in the proximal and the mid LAD artery, normal non-dominant circumflex artery, and severe disease with areas of moderate calcification in the proximal to mid RCA with mild disease in the PDA. He underwent PCI with 3.5 mm× 32 mm EES to the mid RCA and abutting 4.0 mm × 28 mm EES to the proximal RCA, postdilated with 4.0 mm × 15 mm NC balloon with excellent angiographic results.
Antithrombotic Treatment of This Patient
This patient was treated with an LD of aspirin on admission. Given that coronary angiography could not be performed on the same day and there was a clear-cut diagnosis of NSTEMI, he was treated with an LD of ticagrelor 180 mg followed by 90 mg twice daily instead of his prior clopidogrel treatment. It was uncertain whether coronary angiography would be performed within 24 hours and so subcutaneous fondaparinux 2.5 mg was administered. UFH 70 U/kg was administered at the time of PCI. After undergoing PCI, the patient’s long-term ischaemic risk was considered moderate in view of stage 3 CKD and age >65 years but without significant multivessel CAD. His bleeding risk was considered to be high due to the presence of three minor ARC HBR characteristics: CKD with eGFR in the range 30–59 ml/min/1.73 m2, prior ischaemic stroke not meeting the criteria for a major HBR characteristic, and haemoglobin in the range 110–129 g/l for a man. It was planned to continue low-dose aspirin and ticagrelor 90 mg twice daily for 3 months followed by cessation of aspirin and continuation of ticagrelor monotherapy (90 mg twice-daily) for a further 9 months, with a further decision to be made as to whether to continue ticagrelor in the long term or switch back to clopidogrel monotherapy.
Implications of ESC 2023 ACS Guidelines Recommendations for This Case
The management of this patient was consistent with the following ESC 2023 ACS guidelines recommendations:2
- Aspirin is recommended for all patients without contraindications at an initial oral LD of 150–300 mg (or 75–250 mg IV) and an MD of 75–100 mg once daily for long-term treatment. Class 1, level of evidence a.
- Ticagrelor is recommended irrespective of the treatment strategy (invasive or conservative) (180 mg LD, 90 mg twice-daily MD). Class 1, level of evidence b.
- Drug-eluting stents are recommended in preference to bare metal stents in all cases. Class 1, level of evidence a.
However, the management was not consistent with the ESC 2023 ACS guidelines in terms of treatment with an oral P2Y12 inhibitor prior to coronary angiography given that this was performed less than 24 hours after the diagnosis of NSTEMI was made.2 Furthermore, the guidelines recommend parenteral anticoagulation with UFH or, preferably, enoxaparin rather than fondaparinux under these circumstances. However, it was not certain at the time of diagnosis whether coronary angiography would be performed within or more than 24 hours after diagnosis. The patient had HBR and the ESC 2023 ACS guidelines do not provide any recommendation for oral P2Y12 inhibitor treatment before coronary angiography in NSTE-ACS patients with HBR, therefore withholding oral P2Y12 inhibitor treatment for longer than 24 hours should be considered an option in these patients.
The ESC 2023 ACS guidelines provide a default recommendation for 12 months of DAPT in NSTE-ACS patients who do not have HBR but provide support for a range of alternative strategies for shortening the duration of DAPT in those with or without HBR, as follows (Figure 1):2
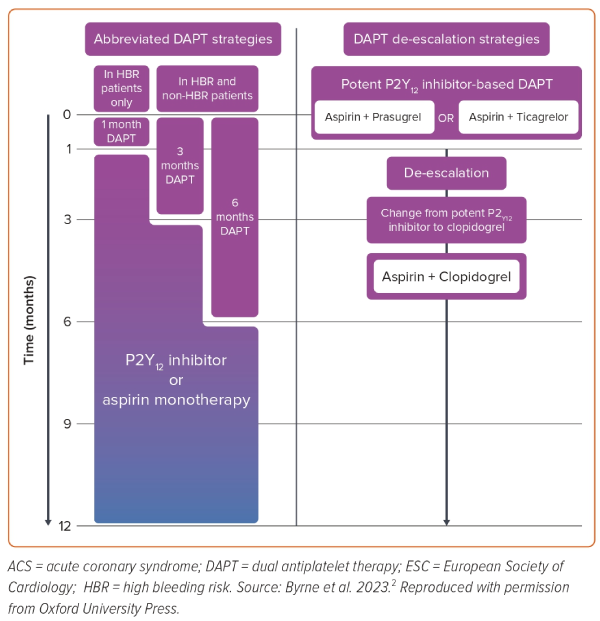
- In patients who are event-free after 3–6 months of DAPT and who are not high ischaemic risk, SAPT (preferably with a P2Y12 receptor inhibitor) should be considered. Class 2a, level of evidence a.
- De-escalation of P2Y12 receptor inhibitor treatment (e.g. with a switch from prasugrel/ticagrelor to clopidogrel) may be considered as an alternative DAPT strategy to reduce bleeding risk. Class 2b, level of evidence a.
- In HBR patients, aspirin or P2Y12 receptor inhibitor monotherapy after 1 month of DAPT may be considered. Class 2b, level of evidence a.
Consequently, for this patient with HBR, either early switching from ticagrelor to clopidogrel may have been considered or cessation of aspirin may have been considered at 1 month rather than 3 months. The guidelines also suggest that cessation of ticagrelor at 1 month may have been considered.
Implications of European Consensus Document for This Case
The European consensus document on antithrombotics makes recommendations for ACS patients undergoing PCI according to HBR status.4 For those without HBR, the default antiplatelet strategy is aspirin and ticagrelor 90 mg twice-daily for 1–3 months, followed by ticagrelor monotherapy for up to 12 months post-ACS and then monotherapy with ticagrelor, clopidogrel or aspirin thereafter unless either (1) there is concomitant polyvascular disease, in which case aspirin plus rivaroxaban 2.5 mg twice-daily is the recommended default strategy, or (2) there is high ischaemic risk and very low bleeding risk, in which case aspirin plus ticagrelor 60 mg twice-daily is suggested although this may be a small proportion of ACS patients. However, in those with HBR, such as in this case, the default strategy is DAPT for only 1 month, consisting of aspirin and either ticagrelor or clopidogrel, followed by SAPT thereafter with ticagrelor, clopidogrel or aspirin. Consequently, the consensus document proposes earlier cessation of DAPT (1 month) than was used in this case of HBR at 3 months post-PCI.
Case Study 3: PCI for ACS with High Bleeding Risk and Atrial Fibrillation
A female patient aged 72 years, with a history of stable angina, hypertension, diabetes, permanent AF and borderline stage 3 CKD was admitted with an episode of severe chest pain lasting 10 minutes. ECG showed AF with satisfactory ventricular rate control and no evidence of ischaemia. High-sensitivity troponin T remained within the normal range. Creatinine was mildly elevated, with eGFR 58 ml/min/1.73 m2 indicating stage 3 CKD, and her haemoglobin was normal. Her CHA2DS2-VASc score was 5. The patient was currently treated with apixaban 5 mg twice daily for thromboprophylaxis in view of the AF and additionally was receiving bisoprolol, amlodipine, atorvastatin and metformin. A diagnosis of unstable angina was made, and she was treated with an LD of clopidogrel as SAPT in addition to apixaban prior to undergoing invasive coronary angiography. This was performed 24 hours after admission via the right radial artery and showed normal left main stem, mild proximal LAD artery disease with moderate diffuse disease of a 2-mm-diameter first diagonal branch, severe stenosis in the mid vessel of the circumflex artery and minor disease of the dominant RCA.
UFH was given as a bolus of a lower-than-standard dose of 60 U/kg because a dose of apixaban had been given 6 hours previously. The circumflex stenosis was predilated with a 3.0 mm × 15 mm balloon followed by implantation of a 3.5 mm × 22 mm EES that was postdilated with a 4.0 mm × 15 mm NC balloon with excellent angiographic results. Rather than giving any aspirin, clopidogrel was switched to ticagrelor 180 mg LD followed by 90 mg twice-daily in combination with apixaban 5 mg twice-daily with a plan to switch back to clopidogrel after 1 month for a further 5 months, followed by apixaban monotherapy in the long term.
Implications of the ESC 2023 ACS Guidelines for This Case
The management of this patient was consistent with the following guideline recommendations:
- During PCI, a UFH bolus is recommended in any of the following circumstances: if the patient is on a non-vitamin K antagonist oral anticoagulant (NOAC); if the international normalised ratio is <2.5 in vitamin K antagonist (VKA)-treated patients. Class 1, level of evidence c.
- In patients requiring OACs, withdrawing antiplatelet therapy at 6 months while continuing OACs may be considered. Class 2b, level of evidence b.
- The use of ticagrelor or prasugrel as part of triple antithrombotic therapy is not recommended. Class 3, level of evidence c.
The UFH dosing strategy was supported by the additional advice in the guidelines to perform PCI without interruption of OAC and, ‘in patients on NOACs, regardless of the timing of the last administration of NOACs, add low-dose parenteral anticoagulation (e.g. enoxaparin 0.5 mg/kg IV or UFH 60 IU/kg)’. Regarding the decision on duration of DAPT, requirement for long-term oral anticoagulation is a major ARC HBR criterion and the patient also had a minor HBR criterion in view of stage 3 CKD. Despite concomitant diabetes, the extent of CAD was not felt to support dual antithrombotic therapy beyond 6 months in view of the technical success of the PCI procedure and limited disease in the unstented major epicardial arteries.
The management was partially consistent with the following recommendation given the high CHA2DS2-VASc score, although no triple antithrombotic therapy was used and clopidogrel was switched to ticagrelor, which is not the preferred strategy:
- As the default strategy for patients with AF and CHA2DS2-VASc score ≥1 in men and ≥2 in women, after up to 1 week of triple antithrombotic therapy following the ACS event, dual antithrombotic therapy using a NOAC at the recommended dose for stroke prevention and a single oral antiplatelet agent (preferably clopidogrel) for up to 12 months is recommended.
The 2023 guidelines no longer provide any recommendation about the use of dual antithrombotic therapy with ticagrelor or prasugrel due to limited evidence.
Given that angiography was performed 24 hours after admission, the management of the patient was borderline inconsistent with the following recommendation:
- Routine pre-treatment with a P2Y12 receptor inhibitor in NSTE-ACS patients in whom coronary anatomy is not known and early invasive management (<24 hours) is planned is not recommended.
Implications of European Consensus Document for This Case
This document did not provide a consensus on antithrombotic management of patients with a baseline indication for OACs, such as AF.
Case Study 4: Percutaneous Coronary Intervention for CCS with High Bleeding Risk
A female patient, aged 76 years, was admitted for elective diagnostic angiography and possible PCI due to stable angina pectoris on mild exertion despite two anti-anginal drugs, bisoprolol and amlodipine. She had a prior history of hypertension and a 22-pack-year history of smoking, which she had stopped 15 years earlier. She had stage 3 CKD with eGFR of 42 ml/min/1.73 m2 and her haemoglobin was 108 g/l, which had remained stable during treatment with aspirin. Her other cardiovascular medication consisted of atorvastatin, ramipril and indapamide. Echocardiography prior to admission had demonstrated mild left ventricular systolic dysfunction with moderate apical hypokinesia.
Coronary angiography demonstrated mild left main stem disease, severe stenosis in the mid LAD artery involving a moderate-sized second diagonal branch, a 50% stenosis in the proximal circumflex which was a small non-dominant vessel, and mild diffuse disease in the RCA. The diagonal artery was treated initially with 2.25 mm × 12 mm balloon dilatation and the mid-LAD stenosis was treated with a 2.75 mm × 18 mm EES, achieving a good angiographic result to the LAD but compromise to the origin of the diagonal artery, which was then treated with a 2.5 mm × 12 mm EES using the T and small protrusion (TAP) technique.
Antithrombotic Therapy Based on Current Guidance
This patient was deemed to have HBR based on both one major ARC HBR criterion (haemoglobin <110 g/l) and two minor ARC HBR criteria (eGFR in the range 30–59 ml/min/1.73 m2; age ≥75 years). The patient continued low-dose aspirin in the long-term and received an LD of clopidogrel 600 mg before proceeding with PCI, followed by an MD of clopidogrel 75 mg once-daily, which was discontinued at 3 months due to the HBR.
This management is consistent with the following ESC 2019 CCS guidelines recommendations:1
- Aspirin 75–100 mg daily is recommended following stenting. Class 1, level of evidence a.
- Clopidogrel 75 mg daily following appropriate loading (e.g. 600 mg or >5 days of maintenance therapy) is recommended, in addition to aspirin, for 6 months following coronary stenting, irrespective of stent type, unless a shorter duration (1–3 months) is indicated due to risk or the occurrence of life-threatening bleeding. Class 1, level of evidence a.
- Clopidogrel 75 mg daily following appropriate loading (e.g. 600 mg or >5 days of maintenance therapy) should be considered for 3 months in patients with a higher risk of life-threatening bleeding. Class 2a, level of evidence a.
Given that this patient had one major and two minor ARC HBR criteria, attenuating the duration of clopidogrel to 1 month could have been considered, according to the following ESC 2019 CCS guidelines recommendation:
- Clopidogrel 75 mg daily following appropriate loading (e.g. 600 mg or >5 days of maintenance therapy) may be considered for 1 month in patients with very high risk of life-threatening bleeding. Class 2b, level of evidence c.
The European consensus document provides recommendations for CCS patients undergoing PCI according to the presence or absence of HBR as well as according to whether or not there is a history of prior MI.4 For CCS patients undergoing PCI without prior MI, the consensus proposes default approaches in those without HBR of either 6 months DAPT with aspirin and clopidogrel followed by SAPT (consisting of aspirin or clopidogrel monotherapy), or 1–3 months DAPT with aspirin and clopidogrel or ticagrelor followed by SAPT with any one of these three; for those with HBR, the consensus proposes 1 month of DAPT with aspirin and either clopidogrel or ticagrelor followed by long-term monotherapy with clopidogrel, ticagrelor or aspirin (Figure 2).
Conclusion
Patients with ACS or CCS present with a diverse range of clinical features, often including a number of comorbidities and risk factors. Managing these patients requires individualisation of the antithrombotic treatment strategy. Recent evidence supports much greater scrutiny of bleeding risk, identifying patients with HBR who warrant attenuated duration of DAPT compared with historical recommendations. Consequently the default strategy of 12 months of DAPT for ACS and 6 months of DAPT following PCI for CCS has been replaced with more nuanced management according to assessment of ischaemic risk and HBR status. The ESC 2019 CCS guidelines and 2023 ACS guidelines provide many recommendations on individualisation of antiplatelet strategy and these are complemented by the European consensus document that draws on two companion network meta-analyses and individual patient data pooled from multiple trials in order to rank the available options and point towards preferred strategies in different scenarios.1,2,4,6