Ventricular arrhythmias (VAs) are a group of potentially fatal electrophysiological alterations, including heart rhythm disorders that cause erratic electrical shocks. They usually occur in patients with heart failure, ischaemic heart disease, hereditary long QT syndrome and catecholaminergic polymorphic ventricular tachycardia syndrome, among others.1,2 These are among the main causes of sudden death in heart disease, with between 50% and 70% requiring medical, pharmacological, electrophysiological and sometimes surgical management.3
The American Heart Association provides guidelines for the treatment of VA.5 Pharmacological therapy includes amiodarone as the drug of choice for patients with structural heart disease or left-ventricular dysfunction, with lidocaine or mexiletine as second-line agents. β-blockers can also be used for patients with structural heart disease or left-ventricular dysfunction. Non-pharmacological therapy options include ICDs for patients with a high risk of sudden cardiac death or who have survived sudden cardiac arrest, and catheter ablation for patients who do not respond to medical therapy or cannot tolerate pharmacological therapy.4,5
Refractory VAs are those that do not respond to standard medical (pharmacological and non-pharmacological) management. The burden of morbidity and mortality associated with VAs that are refractory to medical management can be significant. These arrhythmias can increase the risk of sudden cardiac death, lead to frequent hospitalisations, reduce quality of life and pose challenges in managing patients with advanced heart disease.6
A therapeutic surgical alternative has emerged to reduce the cardiac sympathetic load and interrupt or modify the afferent neural signals involved in altering heart rate, leading to a significant reduction in symptoms.7,8 This technique has improved the quality of life of individuals with VAs refractory to conventional medical management.9–11 Video-assisted thoracoscopic cardiac sympathetic denervation (VAT-CSD) is a minimally invasive surgical technique that seeks to inhibit the sympathetic conduction of the heart, suppressing the transmission of excitatory chemicals, reducing the number of ICD shocks and suppressing the transmission of painful stimuli.5,9,12,13 Published available literature related to this technique has shown a decreased rate of cardiac events up to 54% for patients with VAs.14,15 There is a paucity of literature on VAT-CSD for VAs in Latin America, especially in Colombia. This study aims to describe the experiences of patients with VAs refractory to conventional treatment who have undergone VAT-CSD in Cali, Colombia, in areas with limited resources and access to advanced medical technologies.
The objective was to characterise the perioperative clinical behaviour and outcomes of patients diagnosed with VA refractory to conventional management who underwent VAT-CSD over 10 years in Cali, Colombia.
Methods
Study Design, Setting and Participants
This observational, retrospective study used data from the institutional General Thoracic Surgery Registry at the Fundación Valle del Lili in Cali, Colombia, from 2012 to 2022. The study comprised patients with VAs refractory to conventional treatment who underwent VAT-CSD at this institution. Patients were identified using a standardised numeric code (procedure code number 52.001) used at the institution. From these patients, those with a diagnosis of VA were identified using ICD-10 alphanumeric code I47.
Variables
Information regarding patient demographics (age, sex, weight, height, BMI and comorbidities) and medical management for VA were included.
Primary outcome variables were the number of ICD shocks and visits to the emergency department (ED) before and after VAT-CSD, which were compared to assess the effectiveness of the procedure in terms of symptom control. Secondary outcomes included intraoperative complications, defined as issues or events that occurred during the VAT-CSD procedure, and postoperative complications, defined as issues or events that occurred after surgery and were related to the procedure. Perioperative and postoperative complications were classified according to the Clavien–Dindo system.
Performing the Surgical Technique
The VAT-CSD procedure is performed under general anaesthesia with the use of a double-lumen orotracheal tube for lung isolation. Two bilateral incisions of 5 mm each are made, one for the thoracoscopy port in the fifth intercostal space along the anterior axillary line, and another for surgical instruments in the second intercostal space along the anterior axillary line. The posterior parietal pleura is incised using a monopolar electrocautery hook device to access the thoracic sympathetic chain. The sympathetic chain is then carefully dissected from the stellate ganglion to the sympathetic ganglion of T2.
Subsequently, the lower third of the stellate ganglion (immediately at the upper border of the second rib) and the sympathetic chain below the sympathetic ganglion of T2 (immediately at the upper border of the third rib) are transected using scissors. After nerve transection, ablation of the lower third of the stellate ganglion and the sympathetic ganglion of T2 is performed using a monopolar electrocautery hook device. The VAT-CSD is typically initiated on the left side first, followed by the right side if the patient’s condition remains stable, in consideration of the physiology of the arrhythmias. Finally, the lung is expanded under direct visualisation and air evacuation is carried out using a water trap to prevent pneumothorax. Subsequently, the patient is transferred to the intensive care unit (ICU) for postoperative monitoring.
Our institution, recognised as a regional reference centre for complex cardiac pathologies, has thoracic surgeons who are well trained in the technique of video assisted thoracoscopic sympathectomy. As a result, we have a standardised surgical procedure for thoracic sympathectomies to treat various aetiologies, thus surgical specimens are not routinely analysed to confirm sympathetic denervation for these patients.
Follow-up
All patients underwent ICU management for VAs and received a multidisciplinary approach after surgery. Ten days after hospital discharge, the general thoracic surgical service conducted an outpatient clinic visit to provide surgical healing care and rule out surgical complications through the clinical and radiographical evaluation. For further monitoring of the patient’s progress and for evaluation of symptoms, surgical complications and compensatory sweating, additional outpatient visits were scheduled for 1, 3, 6 and 12 months after the initial visit, conducted by the thoracic surgical service. Meanwhile, during the patient’s recovery period, the Department of Cardiology and Electrophysiology at Fundación Valle del Lili conducted monthly outpatient clinic follow-ups to address cardiovascular pathologies for the first 3 months. After this initial period, the frequency of outpatient clinic visits varied depending on the patient’s clinical needs.
Statistical Analysis
Qualitative variables were reported as absolute (raw) numbers and relative frequencies (proportions). Quantitative variables were reported as measures of central tendency (mean or median) with their respective measures of dispersion (SD or interquartile range [IQR]). The number of ICD shocks in the month before and after the surgery were compared. During the entire follow-up the number of ED visits for the underlying heart disease before and after surgery were also compared. Statistical analyses of paired parametric and nonparametric data were performed according to the data distribution. Statistical significance was set at a p-value <0.05. Data descriptions and analyses were performed using STATA version 15 (StataCorp).
Results
Clinical and Demographics Characteristics
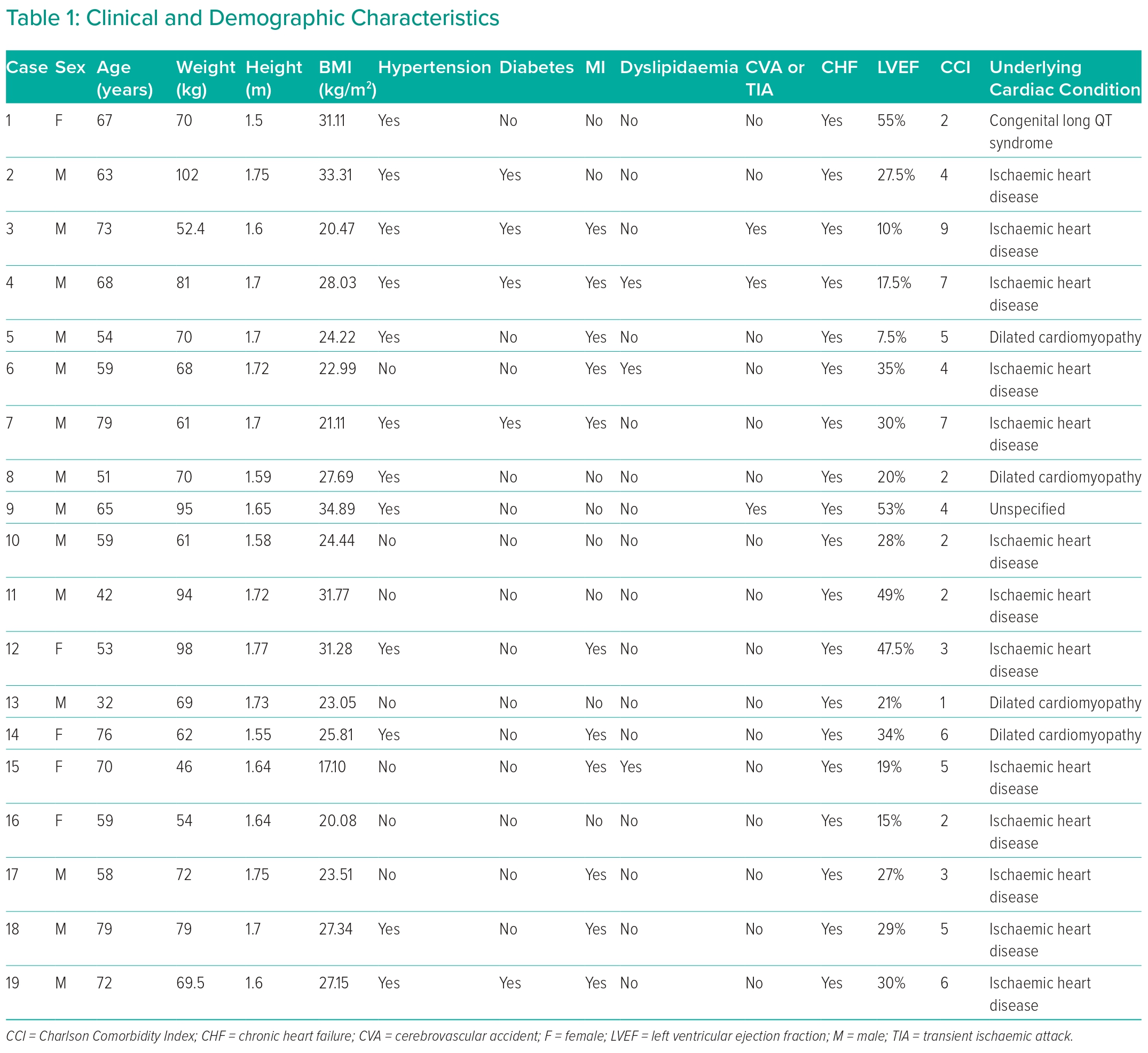
After excluding two patients whose pathologies did not classify as VA, the clinical records of 19 patients who underwent VAT-CSD were evaluated. Among the 19 patients, 73.7% were male (n=14). The mean age was 62 years (SD 12.3), mean weight 72 kg (SD 15.7 kg), mean height 1.66 m (SD 0.75 m) and mean BMI 26 kg/m2 (SD 4.8 kg/m2). The main underlying disease associated with VA was ischaemic heart disease, accounting for 52.6% of cases (n=10). Dilated cardiomyopathy and non-ischaemic cardiomyopathy were present in 15.7% and 10.5% of cases, respectively (Table 1).
Regarding pre-existing comorbidities, all individuals had a diagnosis of heart failure (n=19), 63.1% had hypertension (n=12), 57.8% had a history of acute MI (n=11), 26.3% had diabetes (n=5), 15.7% had cerebrovascular disease or dyslipidaemia (n=3 each) and 10.5% had peripheral vascular disease, hypothyroidism and chronic obstructive pulmonary disease (n=2 each; Table 1). Additionally, 5.2% had obstructive sleep apnoea syndrome, renal disease, or hepatic disease (n=1 for each).
Ejection Fraction and Charlson Comorbidity Index
Median left ventricular ejection fraction, evaluated by echocardiography, was 29% (IQR 19–35%). The risk of comorbidity, assessed using the Charlson Comorbidity Index, showed a mean score of 4.1 points (IQR 2–6). At the time of surgery, 68.4% of patients were enrolled in a heart transplant protocol (n=13). Most patients (94.7%; n=18) had monomorphic ventricular tachycardia; one patient had long QTc syndrome (Table 1).
Symptoms
The most frequent reasons for consultation in the group were increased ICD shocks in 78.9% (n=15), palpitations in 68.4% (n=13), dyspnoea in 63.1% (n=12), chest pain in 36.84% (n=7), syncope or dizziness in 21% (n=4) and the presence of diaphoresis in 10.5% (n=2).
Pharmacological treatment prior to surgery included β-blockers (89.4%; n=17), diuretics (68.4%; n=13), antiarrhythmics including amiodarone (63.1%; n=12), angiotensin II receptor antagonists (52.6%; n=10) and angiotensin-converting enzyme inhibitors (21%; n=4).
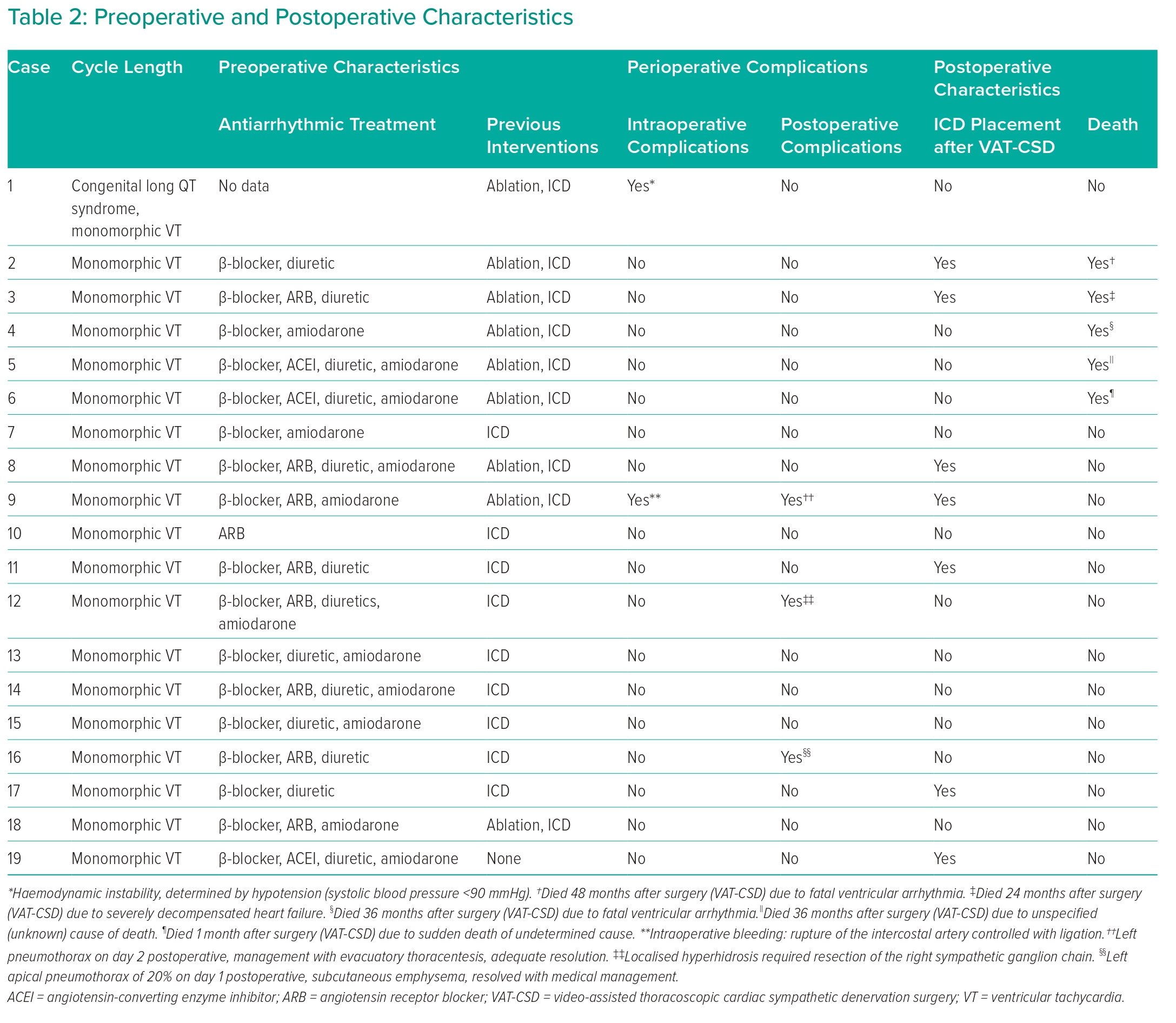
Before surgery 47.3% (n=9) of patients underwent cardiac ablation, with 26.3% (n=5) of these procedures utilising electroanatomic mapping . Additionally, the 94.7% (n=18) of patients had a cardiac device implanted prior to surgery. The most common type was the resynchroniser with cardio-defibrillator (47.3%; n=9). The median duration of symptoms prior to surgery was 397 days (IQR 21–540 days; Table 2).
Surgical Procedure and Intraoperative Characteristics
Surgery was successfully performed bilaterally in 89.4% of patients (n=17). In two patients the surgical procedure was performed unilaterally because of intraoperative haemodynamic instability. The ganglionic level intervened in 100% of the cases was T2 and the mean duration of the surgical procedure was 58.3 minutes (SD, 23.4 minutes).
Perioperative Complications
Perioperative complications were observed, with 10.5% (n=2) occurring intraoperatively, including a case each of VA and tension pneumothorax (5.2% each), but without any intraoperative deaths. Postoperative complications were Horner’s syndrome, compensatory hyperhidrosis syndrome and pneumothorax requiring thoracostomy, each occurring in 5.2% (n=1) of patients. Complications were classified according to the Clavien–Dindo system, with second-degree complications being the most frequent (n=2; 10.5%) followed by one patient each with first-degree and third-degree complications (Table 2).
Primary Outcome Comparison
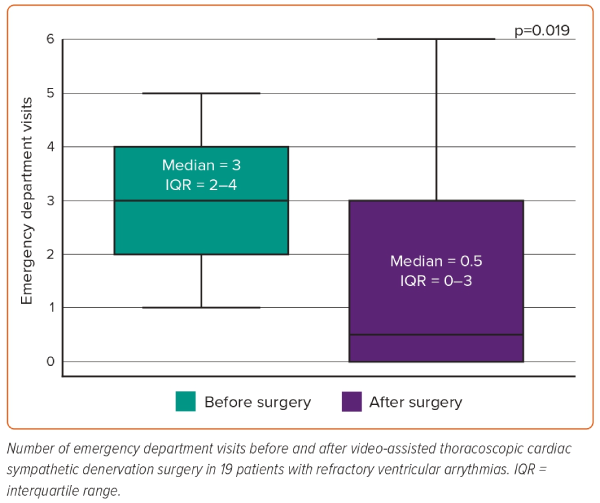
A comparison was made for the number of ED visits related to underlying VA before and after surgery during the study follow-up period in the 19 patients. The median number of ED visits was three (IQR 2–4) and 0.5 (IQR 0–3) before and after surgery, respectively. A nonparametric test of paired data showed a statistically significant difference between the before and after distributions (p=0.019; Figure 1).
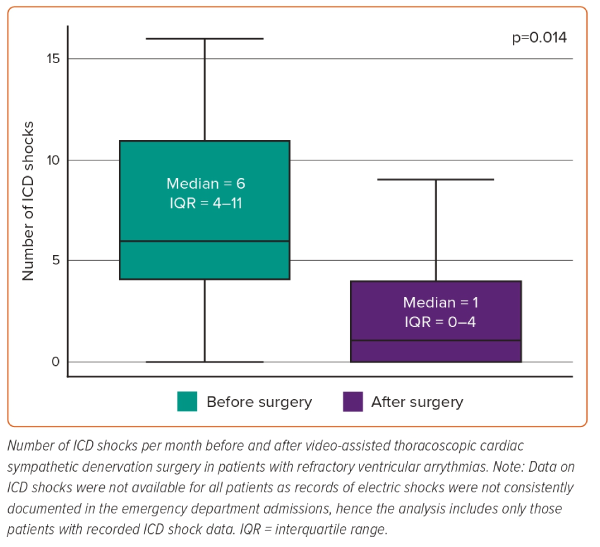
Fourteen patients had information regarding ICD shocks in the month prior to surgery; 17 had information regarding ICD shocks in the month after surgery. The median number of shocks was six (IQR 4–11) before surgery and one (IQR 0–4) after surgery. The distribution of the data was normal and the analysis of variance indicated homogeneity of the variances. A parametric test of paired data demonstrated a statistically significant difference between the two distributions before and after surgery (p=0.014; Figure 2).
Follow-up and Survival
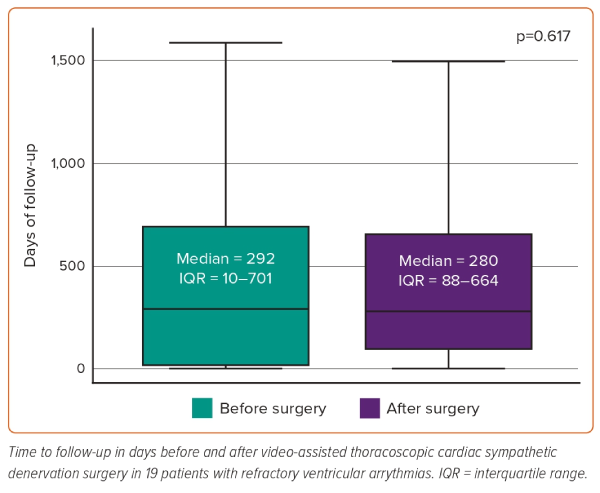
Median follow-up was 292 days (IQR 10–701) before surgery and 280 days (IQR 88–664) after surgery. The distribution of the variable follow-up in days was normally distributed and the analysis of the variance indicated homogeneity of the variances. A parametric test of paired data demonstrated a non-statistically significant difference between the before and after distributions (p=0.617; Figure 3).
During follow-up after surgery 73.7% (n=14) of patients were still alive, with five deaths (26.3%) because of problems related to the underlying disease, including two cases of VA and one case of decompensated heart failure. In the other two cases, the cause was unknown or described as sudden death (Table 2).
Discussion
This case series of patients with refractory VAs who underwent VAT-CSD from 2012 to 2022 was comprised predominantly of male adults (mean age 60 years). The patients had risk factors for cardiovascular disease, including one or more of the following pathologies at the time of surgery: heart failure, hypertension, ischaemic cardiomyopathy and diabetes. Additionally, half of the individuals had a high risk of mortality, as determined by a Charlson Comorbidity Index score >3 points. All patients were under full medical management and there were no other therapeutic options to control their underlying VA.
The findings are consistent with other previously published studies. The case series of 41 patients by Vaseghi et al. showed a significant reduction in ICD shocks during follow-up compared with the 12 months prior.15 In that study, VAT-CSD involved resection of the thoracic sympathetic nerve from the lower third of the stellate ganglion to the T4 ganglion bilaterally in most patients, along with transection of Kuntz’s nerve when present.15 In Colombia there are only two published case series, both from 2019. One, conducted in the city of Bogotá with 20 patients, reported positive results in patients with refractory VAs who underwent VAT-CSD in short- and medium-term follow-up, with 90% resolution of ICD shocks and 100% resolution of arrhythmic storms within 3 months after surgery.16 The other, a case series of six patients in the city of Cali, reported a decrease of symptoms in four patients.17 Other studies have reported effectiveness ranging from 70% to 80% within the first month to the first year after VAT-CSD.18–22
This study evaluated symptom control by measuring the mean number of ICD shocks in the month before and after surgery, as well as ED visits related to arrhythmia before and after VAT-CSD. Significant reductions in the number of ICD shocks in the month after surgery compared with before surgery, as well as a decrease in ED visits after VAT-CSD, were observed. These findings suggest that VAT-CSD may be a viable and safe alternative for improving symptom control and achieving better outcomes in patients with refractory VAs.
In the literature, the main complications associated with VAT-CSD are Horner’s syndrome, nocturnal anisocoria and compensatory hyperhidrosis. The risk of developing these complications can be reduced by using scissors instead of conventional electrocautery during the procedure. However, Horner’s syndrome may still occur because of direct injury to the stellate ganglion or from resection of the lower third of the stellate ganglion, even when scissors are used. Other complications observed in our study included pneumothorax and minor intraoperative bleeding, both of which were managed without the need for reoperation.15,23,24
VAT-CSD is an alternative surgical treatment in patients with VA refractory to medical management, regardless of its aetiology. This study shows that this procedure was safe in this cohort of patients, with a low rate of intraoperative complications, which were mild according to the Clavien–Dindo system. This study has a positive impact on ED visits and the number of ICD shocks and might suggest improvements in patients’ quality of life. Nevertheless, more studies are needed with larger sample sizes and measurement of quality-of-life scores.
The mortality rate in patients undergoing VAT-CSD in the study was 26.3% within a median follow-up of 280 days (IQR 88–664) after surgery. None of the deaths were related to the surgical procedure itself. Similar studies have reported mortality rates ranging from 5% to 40%, with the cause of death mostly attributed to the progression of the underlying disease rather than the surgical procedure.15,16 In our study, three of the five reported deaths were attributed to inadequate control of the underlying arrhythmia disease despite undergoing VAT-CSD.
Other studies have described percutaneous stellate ganglion blockade as a potential alternative to surgical cardiac sympathetic denervation, although it has not been implemented in our institution because of limited experience.25,26 It is important to note that further research with larger sample sizes and longer follow-up periods is necessary to gain a better understanding of the long-term outcomes and mortality rates associated with VAT-CSD in patients with VAs. This would contribute to obtaining more robust evidence of the safety and effectiveness of this procedure in the management of these conditions.
Conclusion
Based on this case series, VAT-CSD appears to be viable and safe. It can be considered as treatment option for patients with VAs who have not responded to medical or ablative treatments. It has shown significant improvement in symptoms with low mortality and perioperative complications. This study suggests a statistically significant reduction in the number of ICD shocks and ED visits after the VAT-CSD procedure. The study population was highly comorbid with a poor survival prognosis based on the Charlson Comorbidity Index. Future studies should explore the potential benefits of VAT-CSD in earlier stages of the disease. Further research with larger sample sizes and inclusion of quality-of-life scores during follow-up evaluations would be valuable for better understanding the outcomes of this procedure. 
Clinical Perspective
- In patients with refractory ventricular arrhythmias, video-assisted thoracoscopic cardiac sympathetic denervation (VAT-CSD) demonstrated statistically significant decreases in the frequency of emergency department visits and ICD discharges.
- The risk of complications associated with the procedure was low.
- VAT-CSD may be a viable therapeutic option for patients with ventricular arrhythmias who have not responded to conventional treatments.