Advances in technique and technology have increased procedural success and improved outcomes in chronic total occlusion (CTO) percutaneous intervention (PCI).1 These challenging cases are made all the more so by the presence of coronary arterial calcification, which is prevalent in CTOs, and an independent predictor of procedural success and complications.2–5 Specific techniques and equipment can be utilised to identify, quantify and overcome the procedural obstacles caused by calcium within CTOs.
Pathophysiology of Calcium in Chronic Total Occlusion
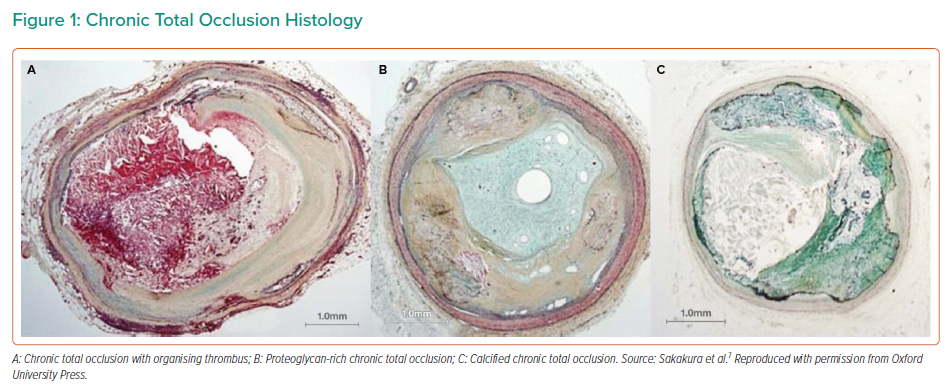
Early in the development of a CTO, the plaque is predominantly soft/lipidic, with organising thrombus, cholesterol-laden cells, foam cells and loose fibrous tissue (Figures 1A and 1B). As the occlusion ages, the composition typically becomes more dense, with fibrous tissue and calcific deposits (Figure 1C).6 Thus, longer duration CTOs are more heavily calcified than those of shorter duration.7
Following coronary artery bypass grafting (CABG), there is accelerated progression of atherosclerosis in the native coronary arteries. Possible mechanisms for this include blood stasis and low shear stress due to competitive flow from the grafts.8 The development of a new CTO in an artery post-bypass is common, occurring in 43% of patients within the first postoperative year.9 CTOs in post-CABG patients are of higher complexity, characterised by more extensive and severe calcification than those in non-bypassed vessels. While calcium is most commonly located at the proximal cap of a CTO, post-CABG it is frequently present in the adjacent proximal and distal vessel, within the CTO segment, and at the distal cap.7 Unique to the post-CABG circulation, the CTO distal cap is exposed, via the bypass graft, to aortic pressure, and thus is more likely to be calcified than in non-grafted occlusions exposed to the lower pressures of the collateral circulation.7 Interestingly, these vessels are less likely to have significant negative remodelling compared with non-calcified non-grafted CTO vessels, possibly due to the calcium acting as a scaffold to prevent vasoconstriction.7
Incidence and Epidemiology of Calcium in Chronic Total Occlusion
Calcium is prevalent in CTOs. On virtual histology intravascular ultrasound (IVUS) analysis, Guo et al. reported that 64% of CTOs contained predominantly fibrocalcific plaque.10 In a retrospective study of 1,476 consecutive CTO PCIs in 1,453 patients in the US, moderate or severe calcium, as assessed by coronary angiography, was present in 58% of occlusions.11 Patients with these calcified occlusions were older and had more comorbidities including diabetes, hypertension, peripheral vascular disease, left ventricular systolic dysfunction and renal impairment.11 Similarly, the PROGRESS registry, Japanese Multicentre registry and a substudy of patients in the Japanese registry undergoing retrograde CTO PCI reported 57–59% of CTOs had angiographically moderate or severe calcification.2,3,12
Success and Safety in Calcific Chronic Total Occlusion
The presence of significant calcification within a CTO has repeatedly been shown to be an independent predictor of PCI success and complications, and thus calcium is a key component of the CTO complexity scores.2,4,5 Other adverse anatomical characteristics often coexist, with calcific occlusions more likely to be longer, tortuous and to have an ambiguous proximal cap.11
In a subset of patients from the PROGRESS registry in whom initial CTO PCI was unsuccessful and who underwent subintimal plaque modification (or a ‘modification procedure’), the prevalence of moderate or severe calcification was 73%.13 In a Japanese cohort of patients who underwent CTCA prior to attempted CTO PCI, 50% of those for whom the attempt was unsuccessful had severe calcification identified on CTCA, compared with 16% of those for whom the procedure was successful.14 When CTO PCI was successful in these cases, severe calcification was associated with higher rates of restenosis and re-occlusion.
The presence of significant calcification hinders the wire-based strategies for crossing an occlusion, with dissection and re-entry techniques often necessary.11 In a Japanese study of antegrade wiring, calcification of the CTO was found to be the most powerful predictor of procedural failure, with an odds ratio of 2.5.15 In addition, moderate or severe calcification is associated with longer procedure duration, higher radiation doses, larger contrast volumes and a higher incidence of major adverse cardiac events (3.7% versus 1.8%) compared with mild or non-calcified CTOs. The most frequent complication is perforation with tamponade due to the requirement for aggressive calcium modifications techniques.11
Imaging of Coronary Calcium in Chronic Total Occlusion
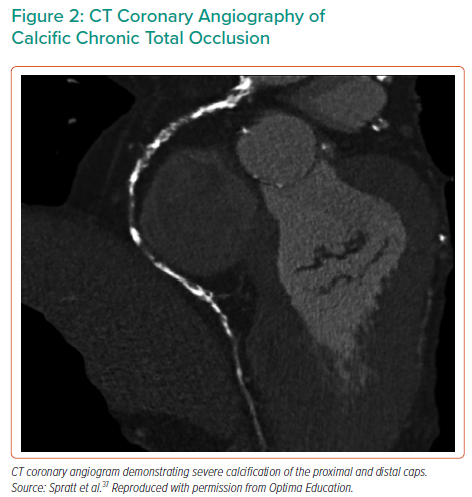
Coronary angiography has a high specificity, but low sensitivity, for detecting calcium, and does not provide information about its morphology or distribution within the plaque and vessel wall.16 CT coronary angiography (CTCA) has higher sensitivity and specificity than coronary angiography for detecting, quantifying and assessing the distribution of calcium, in addition to providing a more accurate measure of occlusion length (Figure 2). While the use of CTCA for procedural planning has been associated with improved CTO PCI success rates, an occlusion length >15 mm and calcification involving >50% of the vessel wall on cross-sectional imaging are independent predictors of procedural failure.17–19
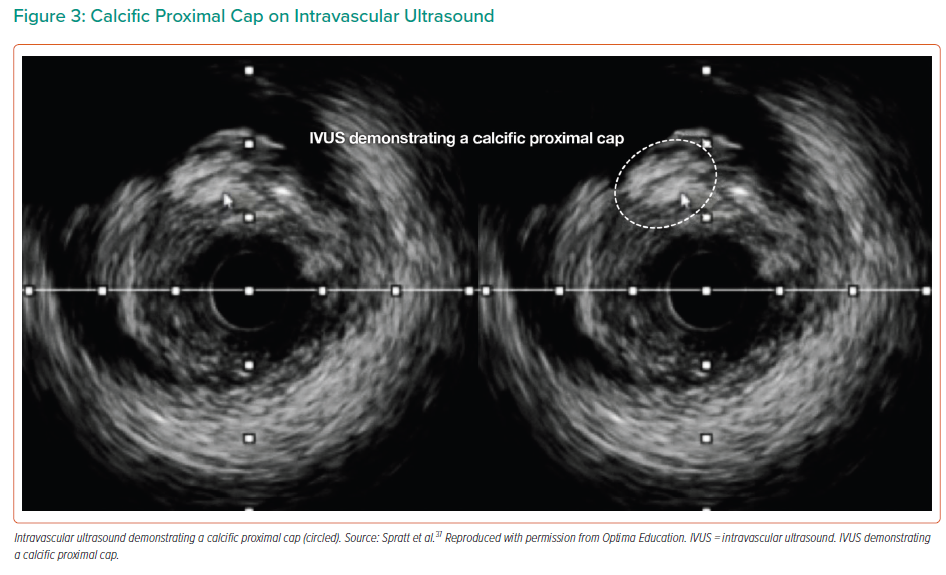
Intracoronary imaging is an essential tool for calcium assessment during CTO PCI. With the desire to avoid extending or expanding extra-plaque tracks through the subintimal space (SIS) by injecting contrast as required for optical coherence tomography, IVUS is usually preferred. In calcified CTOs, IVUS is useful at several stages during the PCI procedure. It can be used to determine the location and the degree of calcification of the proximal cap (Figure 3), when this is angiographically ambiguous. After crossing an occlusion, IVUS can be used to assess the morphology and distribution of calcium, to determine the optimal mode of calcium modification, and then to confirm adequate modification prior to stent implantation. Finally, it can be used for stent optimisation and to measure stent expansion.20 Following dissection and re-entry with extra-plaque stenting, eccentric stent expansion is common due to the displacement of calcific plaque within the vessel structure. In these cases, a pragmatic approach should be adopted as to what constitutes an acceptable stent result, as overzealous post-dilatation has the potential to cause perforation. As in non-CTO PCI, the absolute stent expansion, quantified as minimum stent area is the most important predictor of long-term stent patency.20
Procedural Challenges in Calcific Chronic Total Occlusion Percutaneous Intervention
Lesion Crossing
A procedural set-up with large-calibre supportive guide catheters, a guide extension or an anchor balloon is particularly important when treating calcified CTOs.
Calcium is most commonly located at the proximal cap, resulting in difficulty with wire and microcatheter crossing. Low or medium penetration force wires are unlikely to cross the cap and may deflect into the SIS. If an antegrade wiring strategy is intended, escalation to a high penetration force wire will be required to cross the cap. After the most resistant segment has been negotiated, de-escalation to a lower penetration force wire is recommended to reduce the risk of wire exit. When there is also calcium within the occlusive segment or at the distal cap, further wire escalation and de-escalation may be required.
Coil-based and penetrative microcatheters can facilitate crossing of calcific occlusions. Coil-based microcatheters, such as the Corsair (Asahi Intecc), Mamba (Boston Scientific) and Turnpike Spiral (Teleflex) have internal and/or external coils that allow torque to be applied, to assist propagation through calcific or tortuous segments. The Turnpike Spiral has the addition of distal external threads, which grip resistant plaque to aid crossing, while the Turnpike Gold (Teleflex) has a metallic threaded tip designed to engage and penetrate the proximal cap or calcific plaque. Caution should be taken in long segments of severe calcific disease where the spiral thread or gold metallic tip have the potential to lock into the lesion, making them difficult to remove. The Tornus (Asahi Intecc) is an alternative stiffer metallic catheter with exposed wrapped wires, which is torqued with anti-clockwise rotation.
Intentional balloon rupture, balloon-assisted microdissection or ‘grenadoplasty’, can be useful to facilitate delivery of equipment through the proximal cap or beyond a calcified segment. A small balloon is engaged into the plaque and inflated to high pressure to cause balloon rupture, resulting in hydraulic disruption and weakening of the plaque.
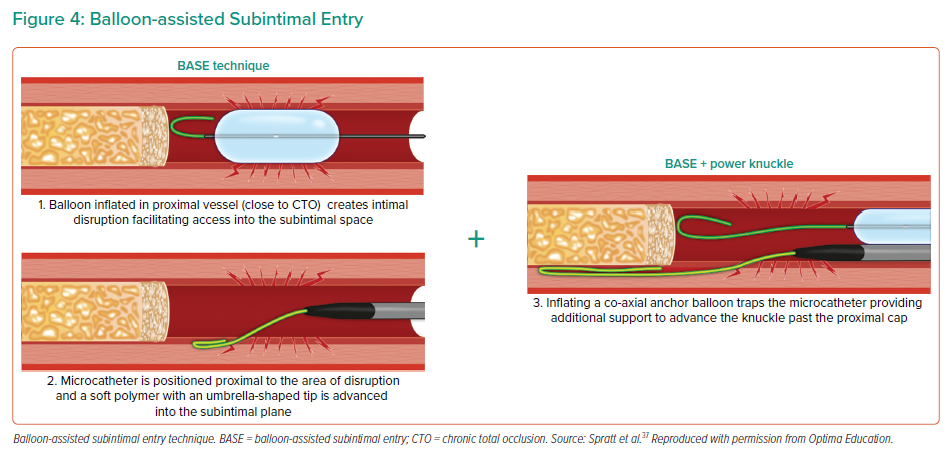
When a wiring strategy with intraplaque crossing is not feasible due to calcification, dissection techniques can be employed by deliberately entering and traversing the SIS, going extraplaque around the calcific occlusion and re-entering the lumen beyond the distal cap. Entry into the SIS can occur while attempting to penetrate the proximal cap or by using specific techniques (e.g. scratch and go, balloon-assisted subintimal entry, power knuckle, contrast-induced hydraulic dissection; Figure 4).21 Antegrade dissection re-entry is usually performed using the CrossBoss/Stingray system (Boston Scientific).
The delivery of equipment and re-entry into the distal lumen can be difficult in the presence of calcium, but can be facilitated by the support of a guide extension and high penetration force wires. In retrograde dissection re-entry, knuckle wires are used to track through the SIS in both an antegrade and retrograde manner, until they become overlapped. The two extraplaque tracks are then connected by performing balloon dilatation to expand the antegrade SIS, allowing passage of a retrograde wire into the antegrade guide extension or catheter.
Plaque Modification
Adequate lesion preparation and calcium modification is essential prior to stent implantation to prevent stent underexpansion, the strongest predictor of early stent thrombosis and restenosis.22,23 This is particularly important in CTO PCI, with longer stented segments increasing the risk of restenosis, and with subintimal stenting following dissection re-entry techniques. Where possible, IVUS should be used to assess calcium modification prior to stenting, and to confirm adequate stent expansion.
Pre-dilatation is routinely performed with non-compliant balloons. When this is inadequate, an ultra-high-pressure balloon (SIS Medical), cutting balloon with longitudinal atherotomes or scoring balloons with an external element can facilitate fracturing and disruption of the calcific plaque.24 While these modifying balloons have been used both intraplaque and extraplaque during CTO PCI, it is anticipated that there will be an associated increased risk of perforation.
The intravascular lithotripsy balloon (Shockwave Medical) delivers sonic pressure waves to selectively fracture calcium deep into the vessel wall and improve compliance prior to stent implantation.25 While it has been demonstrated to be safe and effective in non-occlusive calcific disease, to date there is one case series and several case reports of its use during CTO PCI, both intraplaque and extraplaque following CTO crossing, for in-stent occlusion, to facilitate connection between the antegrade and retrograde SIS during retrograde dissection re-entry, and during modification procedures.26–30
In up to 7% of CTOs, failure to cross with a balloon occurs after successful guidewire crossing.31,32 In such balloon uncrossable lesions, when there is inadequate dilatation with modifying balloons, or when treating a long segment of severe calcium, rotational atherectomy (RA) may be indicated. With a microcatheter across the occlusion, this can be used to exchange for the RotaWire. RA has been reported in case series to be safe and effective during CTO PCI, with the use of a small burr advised to reduce the risk of perforation.32–35 Occasionally, if other techniques for proximal cap crossing fail, RA can be used for cap modification. In this scenario, the RotaWire is advanced as far as possible beyond the cap into the occlusion or SIS, and a focused pecking motion of the burr is used.
RA use in CTO PCI is uncommon, reported in approximately 3% of cases.33,35 Patients requiring RA tend to be older, with a higher prevalence of diabetes, left ventricular dysfunction and previous CABG. In the majority of reported cases, the CTO was crossed by antegrade wiring and RA performed intraplaque, with the indication equally divided between failure to cross with a balloon or inadequate balloon expansion. In a smaller proportion of cases (20–30%), RA was used safely following dissection and re-entry techniques.31,33,35 Cases requiring RA had similar or slightly lower rates of technical success (77–90% versus 85–89% in non-RA cases). Slow/no reflow has been reported in 17%, while perforation rates vary – tamponade requiring pericardiocentesis occurred in 2.6% of RA cases in the substudy of the PROGRESS registry, but did not occur in any RA cases in other, smaller case series.32,33,35
While orbital atherectomy has some potential advantages in non-occlusive disease, the location of the ablative crown 6.5 mm proximal to the tip can be a disadvantage in CTO PCI, particularly in balloon uncrossable lesions. Very few cases have been reported. In the PROGRESS substudy, of 3,607 CTO PCI cases, RA was used in 105, orbital atherectomy in eight cases and both were used in four cases.35
Excimer laser coronary atherectomy generates vapour bubbles to cause the molecular breakdown of tissue. It can be useful to treat uncrossable proximal caps or occlusive segments, balloon undilatable lesions and occlusive in-stent restenosis. It is less available and used less frequently than RA or orbital atherectomy. In a cohort of 18 cases in which excimer laser coronary atherectomy was used alone or in combination with RA during CTO PCI (all of which were antegrade wire escalation), procedure success was achieved in 89%, with no perforations or other laser-related complications.36 It is anticipated that the use of ECLA extraplaque would carry a prohibitive risk of causing perforation of the adventitia.
Conclusion
Calcification in CTOs is common and presents additional challenges during revascularisation of these complex coronary lesions. The presence of calcium predicts lower procedural success rates and a higher risk of complications. Pre-procedural CTCA and intravascular imaging are useful tools to understand the distribution, morphology and severity of calcium. Specialised guidewires and microcatheters, as well as penetration, SIS entry and luminal re-entry techniques, are required to cross calcific CTOs. While the use of each of the balloon-based and atherectomy devices have been reported during CTO PCI, there is limited experience, and some remaining concerns regarding safety and efficacy when used extraplaque.