Coronary calcification has posed challenges to successful revascularisation since the development of percutaneous coronary intervention (PCI).1 Coronary artery calcification worsens clinical outcomes irrespective of the revascularisation strategy.2–4 Despite significant improvements in stent design over the past decades, including an improved ability to deliver a stent, severe calcification continues to impede adequate stent expansion with contemporary PCI. Severe calcification is present in nearly one-third of patients undergoing PCI.5 The degree of coronary lesion calcification impacts stent expansion, which is one of the strongest predictors for in-stent restenosis and stent thrombosis.6–10
Historically, routine use of atherectomy has been reserved for undilatable and uncrossable calcified lesions.11,12 The concept of routine lesion preparation for all heavily calcified lesions prior to stent implantation to prevent stent underexpansion represents a recent change in practice, but is supported by data from intravascular imaging-based studies.6,12–18
Assessment of Coronary Artery Calcification
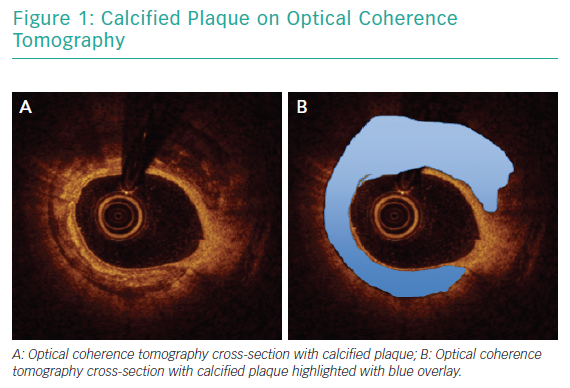
Angiography has been consistently shown to be inadequate for comprehensive assessment of coronary artery calcification.19 In a pivotal analysis of 1,155 coronary lesions, Mintz et al. demonstrated that, while angiography detected calcium in 38% of lesions, intravascular ultrasound (IVUS) was able to detect lesion calcification in 73% of lesions.20 Similar findings were reported in a study that included optical coherence tomography (OCT) in addition to angiography and IVUS.21 Intravascular imaging provides data beyond simple detection of calcified plaque. Lesion morphology, arc of calcification, calcium thickness and length are best assessed with intravascular imaging and can be determinant of the optimal revascularisation strategy.22 Figure 1 illustrates calcified plaque identified on OCT.
Role of Calcium Modification
Mild or moderate calcification is often adequately treated with balloon angioplasty with either a noncompliant balloon, cutting balloon or scoring balloon. When superficial coronary artery calcification is <0.24 mm thick, a balloon alone is often adequate to create fractures in the calcium plate.23 As calcium thickness increases, achieving calcium fracture with balloon angioplasty alone becomes less likely. In patients with an OCT-based calcium score of 4, indicating the presence of calcium thickness >0.5 mm, an arc of calcium exceeding 180o and a continuous length of calcium >5 mm, adjunctive therapies should be used prior to stent implantation.24 Calcium fracture can facilitate improved stent expansion.16,25
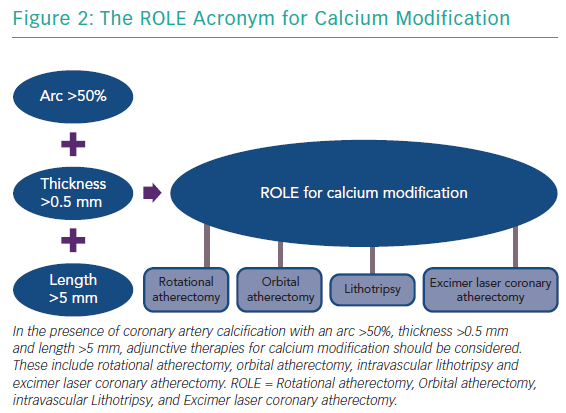
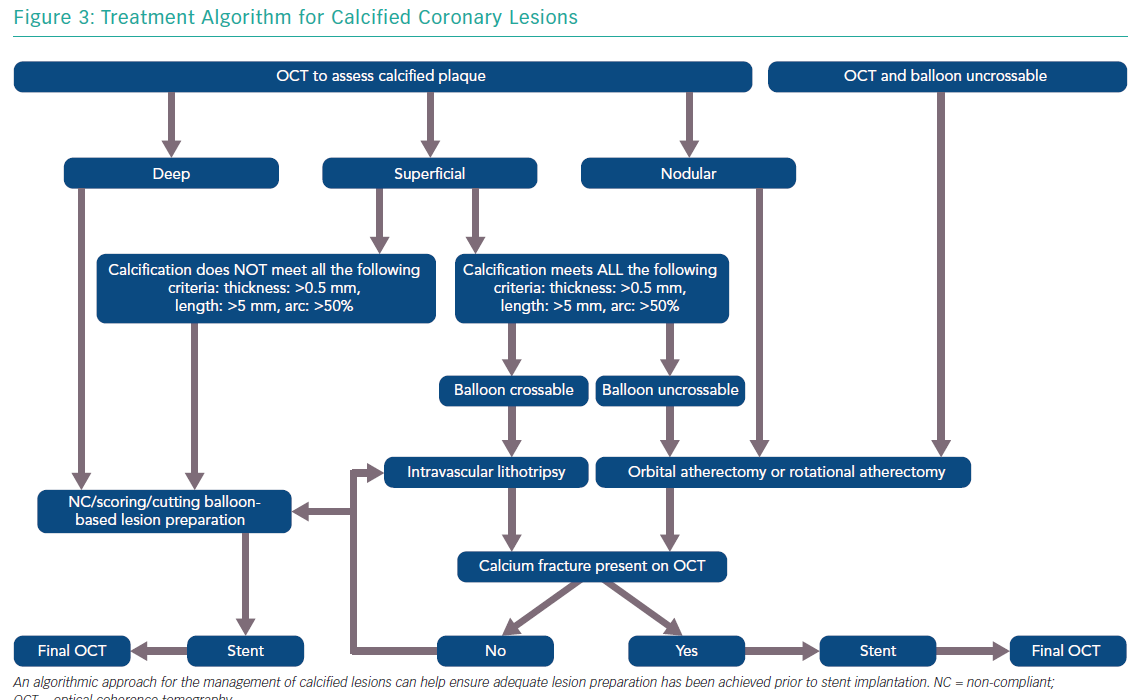
The features of a calcified lesion determine the optimal lesion preparation strategy. The adjunctive therapies that can be utilised for calcium modification as part of PCI can be remembered by the acronym ROLE (Figure 2): Rotational atherectomy, Orbital atherectomy, intravascular Lithotripsy, and Excimer laser coronary atherectomy (ELCA). If a lesion is balloon crossable, intravascular lithotripsy can be used. For lesions that are not balloon crossable, rotational atherectomy or orbital atherectomy are both reasonable options. ELCA can be considered for de novo calcified lesions not amenable to rotational atherectomy, orbital atherectomy or intravascular lithotripsy.26–29 However, the primary role for ELCA in treating calcified plaque is for in-stent restenosis due to an underexpanded stent with significant peri-stent calcification.30,31 An algorithmic approach for the management of calcified lesions can help ensure adequate lesion preparation has been achieved prior to stent implantation (Figure 3). We herein focus on the treatment of calcified plaque with orbital atherectomy.
Orbital Atherectomy
Device Setup and Use
Orbital atherectomy for coronary arteries utilises the Diamondback 360® orbital atherectomy system (Cardiovascular Systems) which incorporates a 1.25 mm diamond-coated eccentrically mounted crown that is advanced over a dedicated 0.012" ViperWire® Advance Coronary Guide Wire (Cardiovascular Systems) with a 0.014" tip. ViperSlide® (Cardiovascular Systems) lubricant is infused during treatment to reduce friction from a specialised pump that is mounted on an intravenous pole. The crown is controlled by a hand-held electric powered single-use handle that has two speeds for treatment: low (80,000 rpm) and high (120,000 rpm). The orbital atherectomy system is compatible with a 6 Fr or larger catheter.
Employing centrifugal forces, the 1.25 mm crown orbits as it advances through a coronary artery, allowing for the continuous flow of blood during atheroablation. This unique mechanism of action combined with an average particle size of debris of 2.04 µm – smaller than a red blood cell – may contribute to low rates of no-reflow and transient heartblock with orbital atherectomy.32 A distinguishing feature of orbital atherectomy is its ability to ablate bi-directionally, potentially decreasing the likelihood for burr entrapment.33
The 1.25 mm crown size is standard for all coronary arteries and can treat larger-sized lumens by either increasing the speed (high speed) or by slowing the rate of advancement to allow an increase in the diameter of the elliptical orbit. By decreasing the traverse speed to 1 mm/second at high speed, the burr orbit can exceed 2 mm in diameter.34 Low speed should be used for all initial passes with orbital atherectomy. Following treatment at low speed, if an adequate endpoint has not been reached, treatment can be escalated to high speed. High speed is not necessary for all cases, and specifically should be avoided in vessels <3 mm in diameter or excessively tortuous vessels to reduce the risk for perforation. The GlideAssist® feature allows for rotation at 5,000 rpm and may be used to aid in the delivery and removal of the device but should not be used for treating a lesion. GlideAssist can facilitate the use of orbital atherectomy by a single operator.35
The goal of lesion preparation is to modify calcified plaque, changing the vessel compliance to facilitate stent expansion.36 The slower the burr is advanced with orbital atherectomy, the larger the orbit is and greater the likelihood of achieving plaque modification as it is a time dependent therapy. The burr should be advanced at 1–3 mm/second. Notably, optimal orbital atherectomy technique differs from the standard rapid pecking motion that is characteristic with rotational atherectomy use.
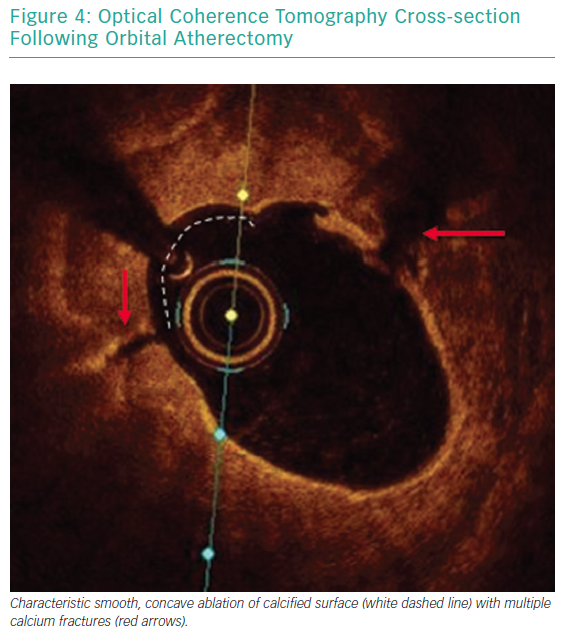
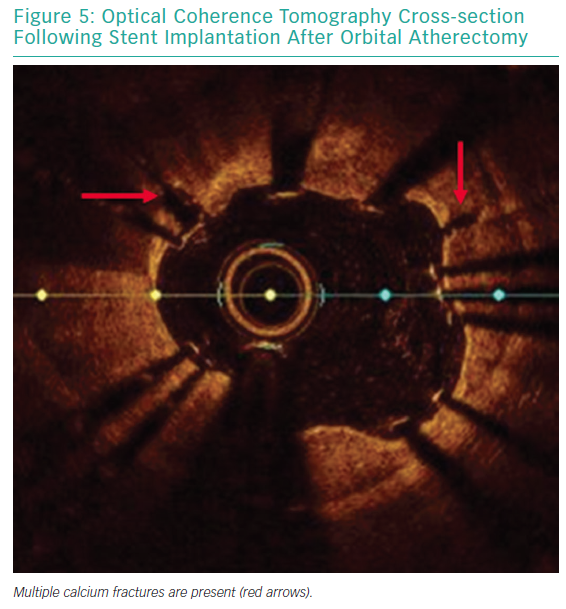
Each pass with orbital atherectomy should be limited to less than 30 seconds. After 25 seconds of continuous treatment, the system emits an audible alert and the crown should be moved to a location outside of the lesion. Rest periods between treatment runs should be for a minimum of duration equal to the preceding run. If the patient becomes symptomatic during treatment, greater rest periods should follow. During atheroablation, pitch changes can be appreciated as the crown engages the hard, calcified plaque. As treatment continues, the pitch change becomes reduced, and tactile resistance decreases.33 Treatment with orbital atherectomy should continue until audible and tactile changes are appreciated, indicating the optimal endpoint has been reached. There is no predefined number of treatment runs that is ideal, and therapy should be adjusted as needed for each specific lesion. While patients have had favourable outcomes following direct stenting after orbital atherectomy, direct stenting in calcified lesions should not be used unless intravascular imaging is performed following lesion preparation confirming an adequate endpoint has been achieved with significant calcium fracture.37 Calcium fracture and the typical smooth, concave ablation seen with orbital atherectomy is readily identified with OCT imaging (Figures 4 and 5).
Clinical Data
The first in-human study of coronary orbital atherectomy of 50 patients treated at two centres in India in 2008 was the Safety and Feasibility of Orbital Atherectomy for the Treatment of Calcified Coronary Lesions (ORBIT I) study, which demonstrated device success of 98% with procedural success in 94% of patients.38 Long-term follow-up was reported and the cumulative major adverse cardiac event (MACE) rate at 3-years follow-up was 18.2%.39 Of note, the ORBIT I investigational study occurred prior to device approval and evaluated four different sized burrs, with only 18.4% of patients having been treated with the 1.25 mm burr that is currently available.38
The pivotal Evaluate the Safety and Efficacy of OAS [Orbital Atherectomy System] in Treating Severely Calcified Coronary Lesions (ORBIT II) study evaluated the impact of orbital atherectomy in 443 patients with severe coronary artery calcification treated at 49 sites in the US from 2010 to 2012.40 The primary safety endpoint, freedom from 30-day MACE, was met in 89.6% of patients and led to device approval in the US in 2013.40 At 3-year follow-up, the overall cumulative MACE rate was 23.5%, and was comprised of 6.7% cardiac death, 11.2% MI and target vessel revascularisation (TVR) in 10.2% of patients with severe calcification treated with orbital atherectomy.41
A real-world multicentre all-comer analysis reported outcomes of 458 patients treated with orbital atherectomy from 2013 to 2015.42 This analysis included a complex cohort of patients that had clinical features that were excluded from the ORBIT II study including unprotected left main disease, low ejection fraction, chronic kidney disease and acute coronary syndrome. Angiographic complications were low with dissection, perforation and no-reflow occurring in less than 1% of cases.42 At 1-year follow-up, the composite rate of major adverse cardiac and cerebrovascular events occurred in 12.6%, with 4.0% all-cause mortality, 1.8% MI rate and TVR rate of 7.5%.43
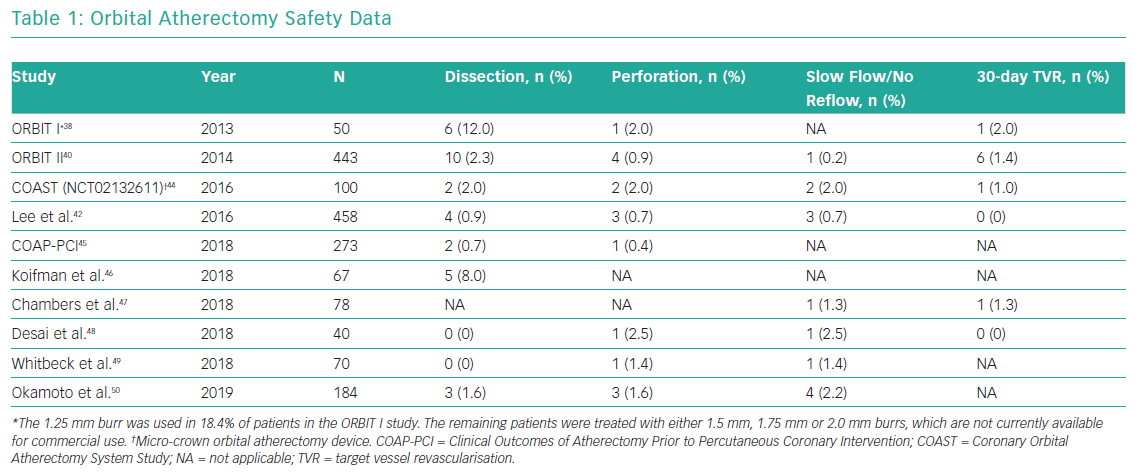
Multiple studies have reported safety data related to orbital atherectomy in both stand-alone orbital atherectomy studies and in observational studies with comparison to rotational atherectomy. The rates of dissection, perforation, slow/no-reflow and 30-day TVR in patients treated with orbital atherectomy are presented in Table 1.
Imaging-based Insights
In a small, multi-centre OCT-based mechanistic study comparing orbital atherectomy with rotational atherectomy in 60 patients, the investigators found that orbital atherectomy creates greater calcium modification in lesions with larger lumen area.51 Calcium fracture following stent implantation occurred in the majority of cases regardless of atherectomy device used, with similar final stent expansion.51 In a separate series where OCT was performed at baseline, following orbital atherectomy, and after stent implantation, OCT demonstrated that in thick calcified lesions, orbital atherectomy was effective at creating post-stent fractures in calcium and facilitating stent expansion.52
Calcium modification is commonly noted on OCT following treatment with orbital atherectomy.53,54 Given the characteristic concave-shaped polishing of the calcium surface with orbital atherectomy, stent strut malapposition may be observed following stent implantation.
Acute stent strut malapposition ≥0.2 mm has been reported as occurring in 7.6% of stent struts at baseline following treatment with orbital atherectomy.53 This typically resolves with neointimal coverage, except when the distance between the stent strut and vessel wall distance exceeds 0.40 mm.53
The impact of orbital atherectomy on the microcirculation is being evaluated in the prospective Comparison of Orbital Versus Rotational Atherectomy Effects On Coronary Microcirculation in PCI trial (ORACLE; NCT03021577), which randomised 20 patients with calcified coronary artery disease to either orbital or rotational atherectomy and will compare the coronary flow reserve and the volume of myocardial necrosis on serial MRI scans.
Future Directions
The Evaluation of Treatment Strategies for Severe CaLcIfic Coronary Arteries: Orbital Atherectomy vs. Conventional Angioplasty Technique Prior to Implantation of Drug-Eluting StEnts trial (ECLIPSE; NCT03108456) is currently enrolling with results expected after 2021. The ECLIPSE trial is randomising approximately 2,000 patients with severe calcific coronary disease undergoing PCI 1:1 to either orbital atherectomy or conventional balloon angioplasty prior to stent implantation. When complete, it will be the largest PCI trial to date in patients with severely calcified coronary artery disease and will assess the role of routine lesion preparation with orbital atherectomy in contemporary PCI and its impact on clinical outcomes.
Conclusion
The presence of calcified plaque is an important determinant of outcomes following PCI. When coronary artery calcification is suspected or known, intravascular imaging should be performed to further assess lesion morphology and need for lesion preparation with adjunctive therapy prior to stent implantation. Orbital atherectomy allows for lesion preparation of severely calcified plaque prior to stent implantation.