The amount of coronary artery calcification increases with age and the presence of cardiovascular risk factors and comorbidities.1,2 Up to 20% of percutaneous coronary intervention (PCI) procedures are challenged by severe calcifications, and coronary calcifications have been shown to be an independent predictor of PCI failure and future adverse cardiac events.3,4 Lesion calcification increases procedural complexity and time. More specifically, calcium localisation (superficial or deep), distribution (focal, circumferential and longitudinal extension) and thickness influence procedural success, stent delivery and deployment.5
Several techniques to treat calcified lesions in native coronary arteries are available, including high-pressure and super-high pressure non-compliant balloons, cutting/scoring balloons, atherectomy devices, both rotational and orbital, and excimer lasers.6 These devices rely on tissue compression and or tissue debulking, and have higher rates of procedural complications, such as dissections, perforations and distal embolisation. Moreover, their success rate is reduced when deep, thick or eccentric calcifications are present, and the induced tissue injury might accelerate uncontrolled neointimal growth and restenosis.6,7 So far, neither specialty balloons nor atherectomy devices have been proved to be superior to high-pressure non-compliant balloons in improving clinical outcomes.8,9
Recently, an alternative way to disrupt calcium has been developed that is based on the lithotripsy concept used to treat kidney and ureteral stones. The Intravascular Lithotripsy (IVL) System (Shockwave Medical) transforms electrical energy into mechanical energy during low-pressure balloon inflation.10 The technology does not rely on direct vascular tissue injury for plaque modification but on sonic waves, which travel from the balloon-based catheter to the surrounding tissue with the intention of safely and selectively breaking both superficial and deep calcium deposits with minimal soft tissue impairment, while improving vessel compliance. In contrast to debulking techniques, the calcium fragments resulting from the IVL therapy remain in situ, reducing the likelihood of distal embolisation. The safety and efficacy of the IVL with minimal vessel injury was demonstrated in prospective single-arm studies, where moderate to severely calcified lesions in peripheral artery disease were tackled; these were the Shockwave Lithoplasty Disrupt Trial for PAD 1 and 2 (Disrupt PAD 1 and 2) and Safety and Feasibility of the Shockwave Lithoplasty® System for the Treatment of Peripheral Vascular Stenosis (BTK) trials.11-14
The Coronary Intravascular Lithotripsy System
The Shockwave Coronary Rx Lithoplasty® Study (Disrupt CAD I), a multicentre, prospective, single-arm study conducted in seven centres in Europe and Australia, was the first to assess the safety and efficacy of the Shockwave Coronary IVL in 60 patients with severe (100%) calcified lesions in native coronary arteries before drug-eluting stent implantation. The balloon was successfully delivered in 59 patients and stent implantation was successful in all cases, with no major procedural complications, such as slow flow, no reflow, distal embolisation or perforation reported. Device delivery was facilitated by pre-dilatation with a small balloon in 37% of patients. Clinical success was 95%, defined as residual diameter stenosis <50% without in-hospital major cardiac adverse events (MACE; a composite of death, MI and target-vessel revascularisation) at 30 days; MACE at 6 months increased to 8.5% with three non-Q wave MI events within the first 30 days and two cardiac deaths.15
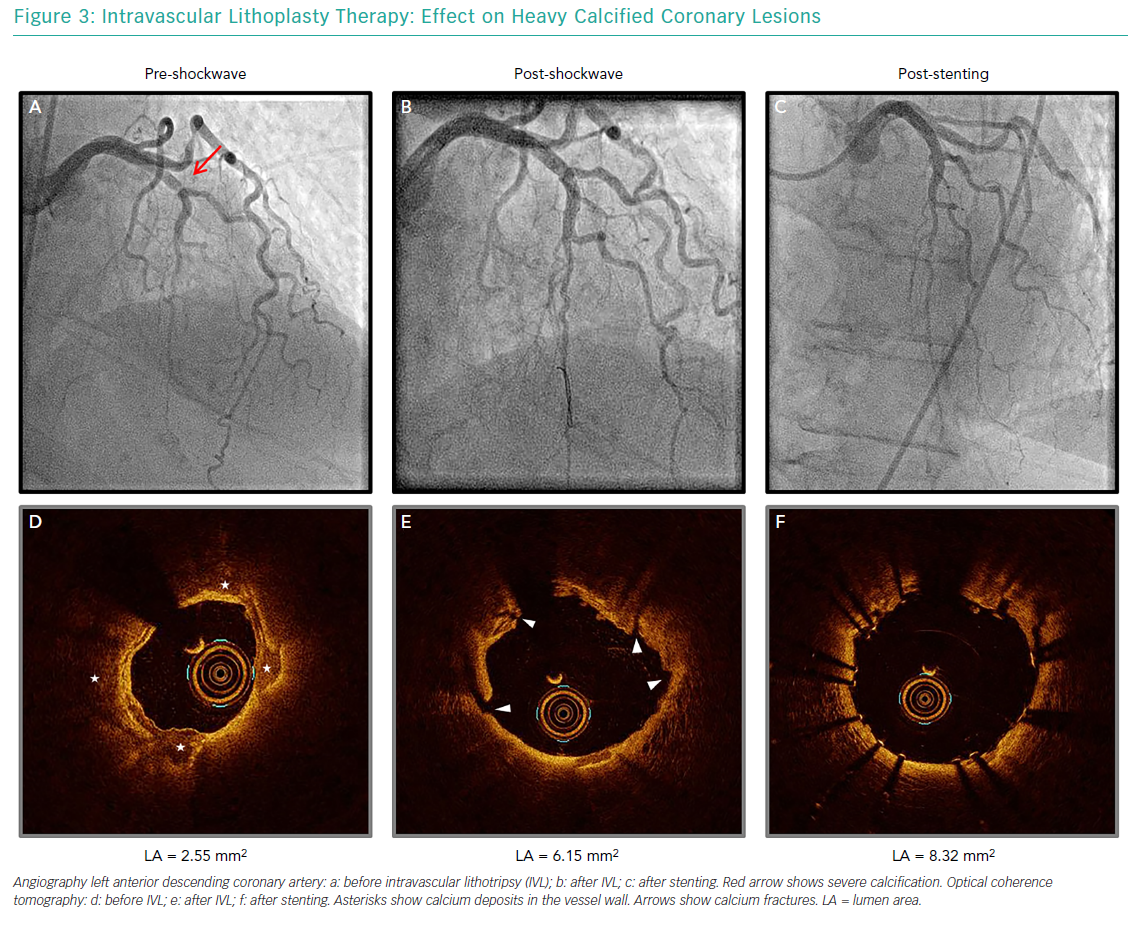
An optical coherence tomography sub-study in 31 patients in the Disrupt CAD I trial confirmed the effects of IVL on the vessel wall.16 Calcium fractures were evident in 42.9% of the lesions and multiple circumferential cracks in the same cross-sectional area were witnessed in more than 25% of the cases, with a higher incidence of fractures in heavier calcified plaques (highest tertile versus lowest tertile; p=0.009). The presence of calcium ruptures allowed to increase acute lumen gain (mean acute area gain = 2.1 mm2) independently of the degree of calcification, enabling successful stent implantation with uniform expansion. Coronary dissections (type B or greater) occurred in four cases during angioplasty and were successfully treated with stent implantation; no other procedural complications were reported. This data provided the first results encouraging the use of the Shockwave Coronary IVL for the treatment of calcified lesions in the coronary vasculature. Following these findings, the device received European CE mark approval in May 2017 for commercial use.
The findings of Disrupt CAD I were further confirmed in a small (n=26) real-world study including patients with both stable and unstable disease, either as an upfront calcium modification technique or as a bailout after suboptimal results with standard balloon dilatation. Angiographic success occurred in all cases (<20% residual stenosis) without procedural complications.17
At present, the results of the Disrupt CAD II (post-market prospective, multicentre, single-arm study), which enrolled 120 patients across Europe, are pending. More recently, the Disrupt CAD III study was announced. The study will aim to enrol 392 patients in 50 centres across the US and Europe with the intention to obtain Food and Drug Administration (FDA) approval.
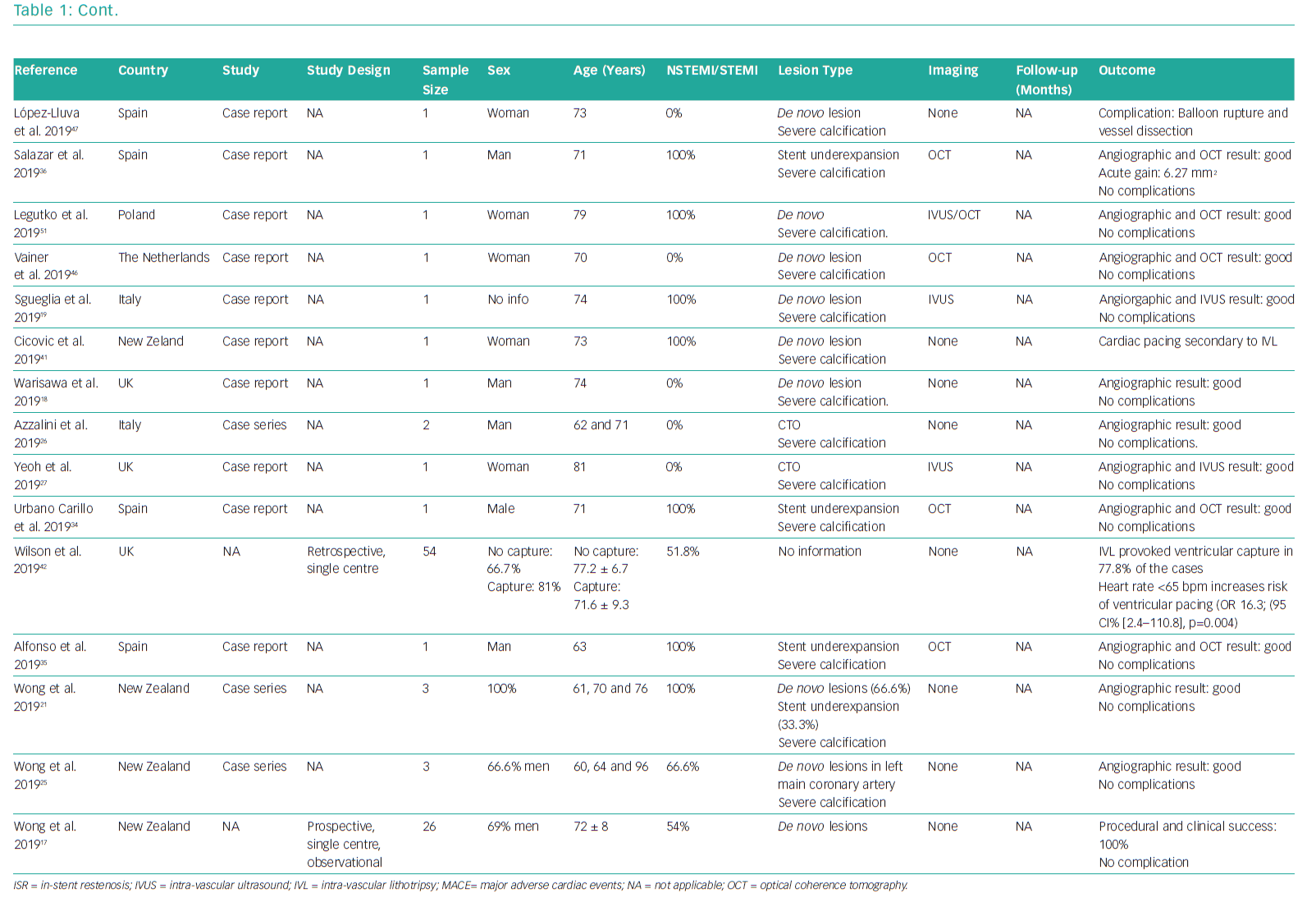
The available studies and case reports on the use of coronary IVL are summarised in Table 1.
The System
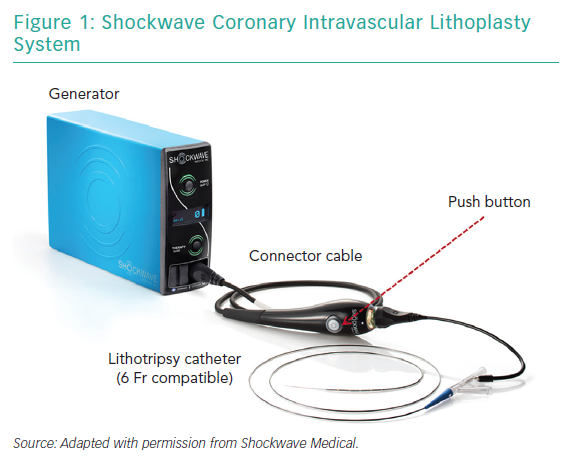
The Coronary IVL System consists of a portable, rechargeable generator, a connector cable with a push button to allow manually controlled delivery of electric pulses, and a 6 Fr compatible, rapid-exchange, semi-compliant balloon catheter to be used following standard angioplasty practice over a 0.014" guidewire (Figure 1).
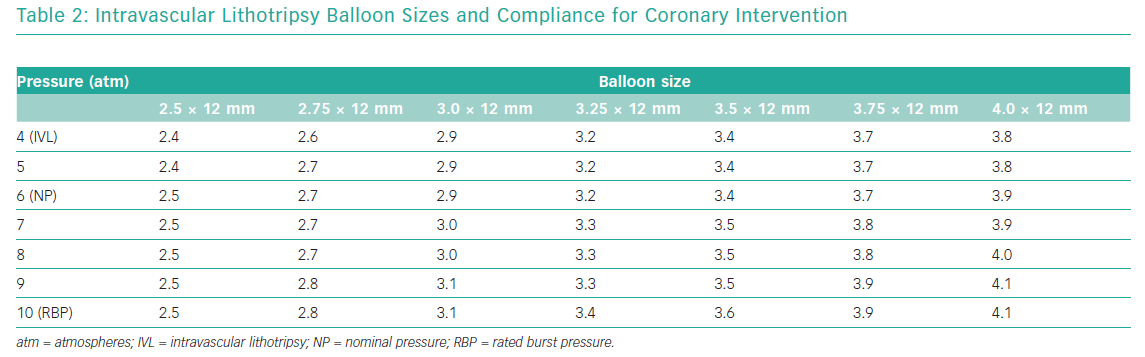
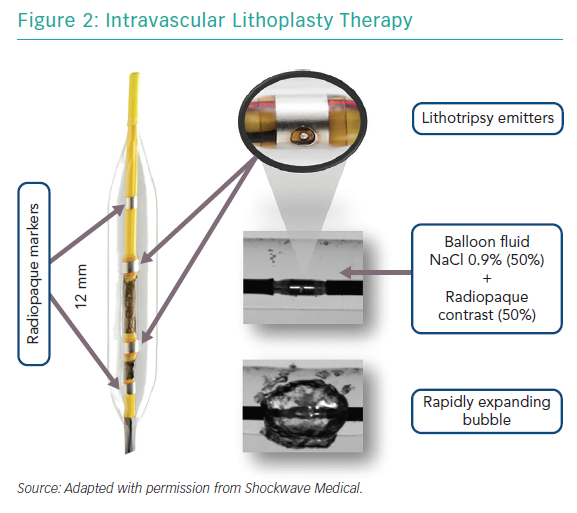
The semi-compliant balloon integrates two radiopaque lithotripsy emitters 6 mm apart and two conventional markers at the proximal and distal edges of the balloon. These emitters receive electrical pulses from the generator vaporising the fluid (a standard mixture of 50% NaCl 0.9% and 50% radiopaque contrast) within the balloon and creating a rapidly expanding and collapsing bubble. This bubble can transmit unfocused circumferential pulsatile mechanical energy into the vessel wall, in the form of sonic pressure waves equivalent to approximately 50 atmospheres (atm). The balloons are available in diameters ranging from 2.5 mm to 4.0 mm with a standard length of 12 mm (Table 2); their crossing profiles range from 0.043" to 0.046" (Figure 2).
The IVL therapy consists of a series of 10 pulses (1 cycle) or 10 seconds (1 pulse per second). The number of therapies needed per lesion will depend on lesion resistance; however, the maximum number of pulses to be delivered by each individual catheter is limited to 80 pulses (eight cycles).
Intravascular Lithotripsy Procedure
The IVL procedure does not require high-level additional training for interventional cardiologists. The shockwave balloon must be sized accordingly to the reference vessel diameter (ratio 1:1), placed in the target calcified lesion and inflated up to 4 atm to ensure apposition to the vessel wall; the lithotripsy emitters are then activated to deliver the acoustic pulses by pushing the button on the connector cable. Once a cycle of 10 pulses has been delivered, the balloon can be inflated up to 6 atm (nominal pressure) to increase balloon compliance and to assess symmetrical expansion, confirming calcium modification. Next, the balloon is deflated carefully to allow small air bubbles to escape. The previous steps must be repeated for each intended IVL cycle and at least two IVL cycles are recommended to treat the target area. For the treatment of lesions longer than 12 mm, the catheter needs to be repositioned and overlapping treatment areas might occur. See Supplementary Videos 1–4.
Due to the slightly higher profile of the shockwave catheter, pre-dilatation with standard balloons might be necessary in some cases to facilitate deliverability and positioning, especially when lumen reduction is severe. Notwithstanding this, the balloon allows the use of guide-catheter extenders and buddy-wire support.18 Furthermore, although the system is labelled 6 Fr compatible, it could be used with a 5 Fr guiding catheter where the radial artery is small.19 The use of dilatation with non-compliant balloons after IVL, although not mandatory, could be considered to expand the lumen further. Moreover, aggressive plaque modification devices such as cutting/scoring balloons or atherectomy could be used as adjuvant therapy in challenging lesions to improve results.20
Potential Uses
At present, the instructions for use of the Shockwave Coronary IVL System restrict its use to lesion preparation in native coronary arteries (Figure 3). Given it is assumed to be safer than previous approaches, the number of case reports and small case series reporting on its use in more challenging scenarios is increasing.
Acute Coronary Syndromes
Calcified lesions in culprit vessels are common in patients presenting with acute coronary syndromes (moderate calcification is found 26.1% of these patients and severe calcification in 5.9%), and their presence is a strong predictor of definite stent thrombosis (HR 1.62; 95% CI [1.14–2.30]; p=0.007) and target lesion revascularisation (HR 1.44; 95% CI [1.17–1.78]; p<0.001).1 The Disrupt CAD study included only patients with stable and unstable angina. Although there is not enough evidence to support the use of the IVL during primary PCI, early experience has shown favourable results.17,21
Unprotected Left Main Calcified Stenosis
PCI has become an option for the treatment of left main (LM) disease, with a class IA recommendation for patients with a SYNTAX score ≤22 and class IIA for a SYNTAX score 23–32.22 Calcification increases procedural complexity and therefore the risk of complications. Although atherectomy has previously been proposed as a valid and feasible option, the high-risk profile of those patients means safer approaches would be welcome.23,24
The Coronary IVL System, with controlled pulses delivered under low pressure, might potentially improve plaque modification with a lower risk of vessel closure, perforation or embolisation.25
Chronic Total Occlusions
Moderate to severe calcification is frequently found in chronic total occlusions, and debulking devices are usually avoided because the procedure is difficult and has a high risk of complications; where standard balloons fail, the Coronary IVL System might be useful in facilitating lumen dilatation and communication with the subintimal space.26,27
Stent Underexpansion due to Underlying Calcification
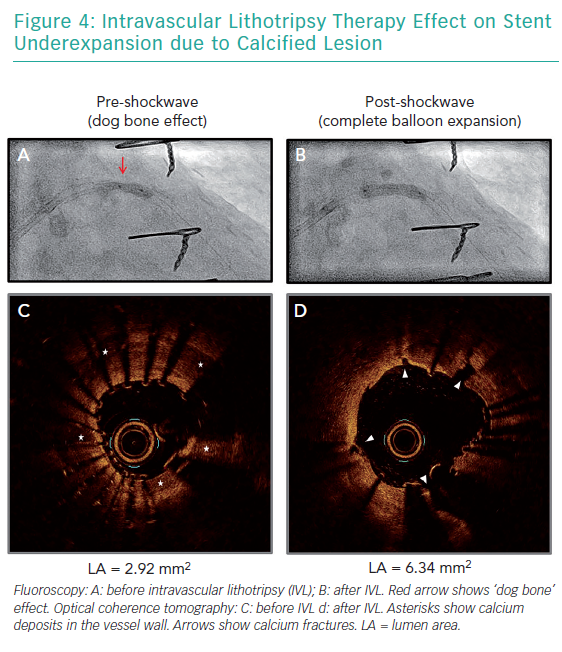
Although the technique has been developed to treat calcified lesions in native coronary arteries before stenting, patients with severe stent underexpansion because of heavy calcification are at a higher risk of stent failure and future adverse events. Until now, undilatable lesions in previously stented segments have been courageously approached with debulking devices such as cutting balloons and atherectomy, with unpredictable results and risks of procedural complications and stent damage.28–31
Of note, the effectiveness of those techniques is limited by the presence of metallic struts, and deeper calcifications therefore remain unaffected. The circumferential sonic waves of the Coronary IVL System, conversely, have the advantage of extending beyond strut layers and fracture deeper calcium deposits (Figure 4). Several case reports have supported the use of the technology for optimising stent expansion without complications.32–39
Of note, the efficacy of the system in segments with multiple layers of stents has not been demonstrated and its impact on stent backbone/polymer integrity and drug elution is still unknown. Nonetheless, at present, there are no alternative percutaneous options for patients left with underexpanded stents due to heavy calcification.
Effects on Cardiac Rhythm
Electric signals similar to pacing spikes on the electrocardiogram (ECG) tracing during pulse-delivery have been described.17,40–42 These so-called ‘shocktopics’ and asynchronous cardiac pacing have been reported in up to 77.8% of the cases, with a 16-fold increased risk in patients with a heart rate <65 BPM. Cardiac pacing has not been linked to any specific number of IVL cycles or coronary artery anatomy, although its frequency is higher when either the left anterior descending artery or the right coronary artery are treated.42
The exact mechanism behind this phenomenon is still unclear. A potential explanation might be that the transformed mechanical energy reaches and couples with the cardiac conduction system producing ectopic atrial and or ventricular captures.43 Although no relevant clinical events have been reported, it warrants paying special attention to ECG and aortic pressure waveforms changes during IVL administration; the resulting VOO pacing mode is theoretically pro-arrhythmic (potential R on T phenomenon) and, until further data become available, pacemaker carriers should be assessed for inappropriate device sensing during the IVL cycles and to ensure correct pacing function post-procedure. Further investigation in the matter will be provided by the substudies in the Disrupt CAD III trial.
Challenging Lesions
Some lesions might respond better to IVL therapy than to other plaque modification approaches. Reports have pointed out the usefulness of the IVL therapy on creating calcium fractures as assessed with intravascular ultrasound, and achieving optimal stent expansion in undilatable lesions that have been resistant to specialty balloons and rotational atherectomy.44–46
In contrast, some lesions might not be suitable for IVL treatment or may remain resistant after the application of all 80 pulses. Severe tortuosity or angulation, critical lumen reduction, plaque indentation into the lumen and a very low vessel expansion compliance (small vessels and multiple stent layers present), could impact balloon deliverability and positioning. Up to 46% of the lesions might also require dedicated lesion pre-dilatation and/or post-dilatation with non-compliant balloons or could benefit from adjuvant lesion preparation with conventional devices such as specialty balloons or atherectomy to either facilitate balloon delivery or increase calcium compliance after lithotripsy therapy.17,45
While balloon rupture is uncommon, it can cause vessel complications. Case reports have described sudden IVL balloon burst during lithotripsy therapy with important vessel dissection; however, it is fair to highlight that either critical stenosis or severe vessel tortuosity were present, which suggests that, in some anatomies, the IVL system might not be suitable or should be used with caution.47,48
Furthermore, vessels with a diameter >4 mm (maximum shockwave balloon size) or important plaque eccentricity preclude appropriate IVL balloon apposition to the vessel wall, and may reduce the efficacy of the therapy. More data are needed on the specific efficacy of IVL in concentric versus eccentric lesions. In the Disrupt CAD study, 22% of the patients had eccentric plaques; nevertheless, overall device success was 98%.15
Moreover, performing intracoronary imaging where important coronary calcification is suspected during the angiographic assessment could help to accurately assess calcium distribution, localisation and thickness. The use of intracoronary imaging before and after lithotripsy therapy could not only assist the appropriate selection of IVL balloon sizes but also potentially help to identify IVL responders and identify patients who might need adjuvant therapy from other plaque-modification devices .
Further clinical data are needed to assess the implications of patient, vessel and lesion characteristics on the effectiveness of this therapy, the need for further adjuvant therapy with conventional devices and potential complications.
Conclusion
The Coronary IVL System is a promising new treatment modality to tackle moderate to severe calcified coronary lesions, with a high rate of success and a low risk of complications. Larger studies and longer-term clinical data are needed to confirm the safety and efficacy of this technique with special attention to the effects on cardiac conduction and vessel healing response. Randomised controlled clinical trials are required to evaluate its superiority against currently available calcium-modifying devices.
Supplementary material