The prevalence of calcified coronary lesions encountered in daily percutaneous coronary intervention (PCI) is set to increase with the growing prevalence of predisposing factors such as hypertension, ageing and diabetes.1 Calcified lesions lead to sub-optimal PCI outcomes by limiting the crossing of lesions, altering drug elution kinetics and interfering with optimal stent expansion, and they are also associated with poorer clinical prognosis.1–6 Common technologies to treat calcified plaque, such as rotational or orbital atherectomy, are associated with increased periprocedural complications without clear clinical evidence of efficacy.7 There is thus an unmet need for effective and safe methods to prepare calcified lesions and improve PCI outcomes.
Intravascular Lithotripsy: Technical and Scientific Overview
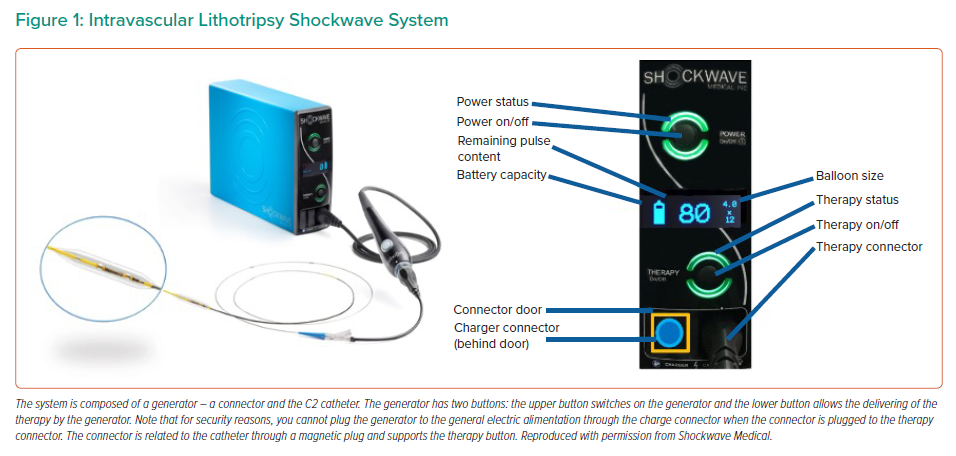
Intravascular lithotripsy (IVL) is a new vessel preparation technique for calcified coronary artery lesions that creates multiplane micro/macro fractures in the calcified plaque before stenting or allowing for an improved stent expansion.8 The C2 Shockwave Medical Coronary IVL system (Shockwave Medical) consists of three components: a generator; a connector cable and a sterile catheter incorporating the lithotripsy emitters enclosed in a semi-compliant balloon (Figure 1). IVL emitters produce electric sparks that create vapour bubbles in the surrounding fluid medium in the integrated balloon. IVL produces low levels of electric energy, leading to the formation and rapid expansion of vapour bubbles, resulting in acoustic pressure waves that radiate circumferentially and transmurally in an unfocused manner. These acoustic pressure waves interact with high-density tissues such as calcium without affecting soft tissue. This interaction disrupts the calcium by creating micro-macro fractures, and increases vessel compliance.8
The catheter, available in 2.5–4.0 mm diameters, is programmed to deliver 10 pulses in sequence at a frequency of 1 pulse/second for a maximum of 80 pulses per catheter. The low pressure inflation avoids the barotraumatic vessel wall injuries related to high pressure inflation.8,9 The role of the fluid-filled integrated balloon is to facilitate efficient transmission of shockwave energy to vascular tissue by several mechanisms: creation of the spark which requires ions, adequate interface with similar acoustic impedances, avoiding thermal injury, and shielding the emitters from direct contact with the arterial wall.8 Compared with atherectomy, the IVL acoustic burst penetrates deeper into the arterial wall to generate multiplane longitudinal fractures without affecting healthy tissue.
The coronary IVL system has received both the CE Mark and FDA approval. IVL has been studied in the Disrupt CAD clinical trials, including the pivotal Disrupt CAD III study. The most recent data confirms that IVL is a safe procedure with a high success rate – primary safety and effectiveness endpoints were achieved in 92.7% and 92.4% in the pooled analysis of all Disrupt clinical trials. At 30 days, the rates of target lesion failure, cardiac death and stent thrombosis were 7.2%, 0.5% and 0.8%, respectively.10 However, patients in such trials are highly selected and are not representative of daily clinical practice.
The aim of this review is to focus on best IVL practice based on the authors’ 3 years of experience and the latest scientific data from Disrupt CAD.
Intravascular Lithotripsy Catheter Preparation
As the acoustic shockwaves are propagated through fluid and are impaired by air, the first step is to wash out the air in the catheter using a standard technique. This stage is essential to ensure optimal transmission of the sonic wave into intimal and medial layers. We recommend filling a syringe with 5 cc of 50/50 saline/contrast medium and connecting the syringe to the inflation port on the catheter hub. Pull vacuum at least three times to allow the fluid to replace the air in the catheter. Disconnect the syringe and connect the inflator device with 10 cc of 50/50 saline/contrast medium to the inflation port, ensuring no air is introduced into the system.
Supplementary Material Figure 1 shows a step-by-step guide to therapy delivery.
Intravascular Lithotripsy Target Lesion Selection: Concentric or Eccentric Calcification?
As the IVL emitters generate a circumferential acoustic wave, arterial circumferential calcification is the most suitable target for IVL. Moreover, part of the acoustic wave is transferred across the calcified plaque and reflected, interacting with the opposite side of the lesion in a process known as spallation, which increases the procedure’s efficiency.11 Circumferential calcification modification increases vessel compliance and allows for full symmetrical stent expansion. For these reasons, circumferential calcification was an inclusion criterion in the Disrupt CAD trials, defined by the presence of fluoroscopic radio-opacities without cardiac motion involving both sides of the arterial wall, or by the presence of ≥270 degrees of calcium on at least one cross-section on intravascular imaging.12–14 Other criteria were a diameter stenosis ≥70%, native coronary artery lesion length ≤40 mm and heavy calcification, defined as calcification within the lesion on both sides of the vessel assessed during angiography.14
There is evidence that IVL may also be appropriate for eccentric lesions as suggested by a post-hoc analysis in a pooled patient population with eccentric calcified lesions (identified by an independent core lab) in Disrupt CAD I and II.15 Eccentric lesions were defined as a stenotic lesion that had one of its luminal edges in the outer one-quarter of the apparently normal vessel lumen whereas concentric lesion has the same criteria but involving both luminal edges.
We found a high procedural success rates, defined as a residual stenosis of <50% after stenting without intra-hospital major adverse cardiovascular events (MACE) of 93.6% and 93.2% in eccentric and concentric lesions, respectively. There was no difference in vascular complications and clinical outcomes according to the definitions of coronary artery disease (CAD). No difference in mean target delivery pulses between the two groups have been noted for reaching procedural success. However, over 3 years of IVL use in clinical practice, several users have suggested that more pulses may be required to modify calcium in eccentric lesions due to the emitter being at a greater distance from the plaque and the lack of wave reflection compared with concentric lesions. From our experience, we recommend using a 1.1 non-compliant (NC) balloon post IVL therapy to determine the effectiveness of the IVL procedure and to assess the need for an additional IVL catheter.
Intravascular Lithotripsy Therapy Application
The catheter diameter should be selected at a 1:1 ratio relative to the target-vessel diameter and inflated at a sub-nominal pressure (4 atm). Proper apposition of the catheter to the arterial wall is necessary for an adequate fluid/tissue interface to optimise acoustic energy transfer. As noted above, there are several mechanisms responsible for the disruption; in addition to spallation squeezing, cavitation and plaque fatigue that all play a role, as detailed in a recent review.11 The estimated peak pressure of the wave is 50 atm. Notably, the wave and not the balloon generates the disruptive force. This has several advantages – it allows low-pressure balloon inflation which reduces the risk of barotrauma, vascular dissection and perforation and post-dilation after IVL for residual stenosis before stenting (defined in Disrupt CAD III as a residual stenosis >50%) is often unnecessary. In the Disrupt CAD III study, post-dilation was used in only 20.7% of cases.14
In clinical practice, most operators recommend performing an NC balloon after dilation only if there is an incomplete expansion of IVL catheters of more than 30%. It remains unclear how many pulses need to be delivered to ensure good lesion preparation. One catheter can deliver a maximum of 80 pulses.
On average, two IVL catheters were used in the Disrupt CAD I and 1.2 were employed in Disrupt CAD II and III. In the Disrupt pooled analysis, the mean number of pulses were 74.7 ± 42.7 and 1.3 ± 0.6 catheter was used.10 According to protocol in Disrupt CAD I, II and III, we assume that a minimum of 20 pulses delivered to the target lesion should be the lowest threshold required for pulse delivery if there is no residual footprint on the balloon.
Use of Endovascular Imaging
Endovascular imaging may be useful for pre- and post-procedural evaluations.
Pre-procedural Evaluation
Coronary angiography often underestimates calcium.16 In our experience, optical coherence tomography (OCT) is the preferred imaging modality due to its high spatial resolution and ability to measure calcification thickness. Intravascular ultrasound imaging (IVUS) is a valid alternative. In an in vivo sensitivity assessment of 440 lesions, OCT detected calcium in 76.8% and IVUS in 82.7% of lesions, whereas angiography identified calcium in only 40.2% of lesions.17
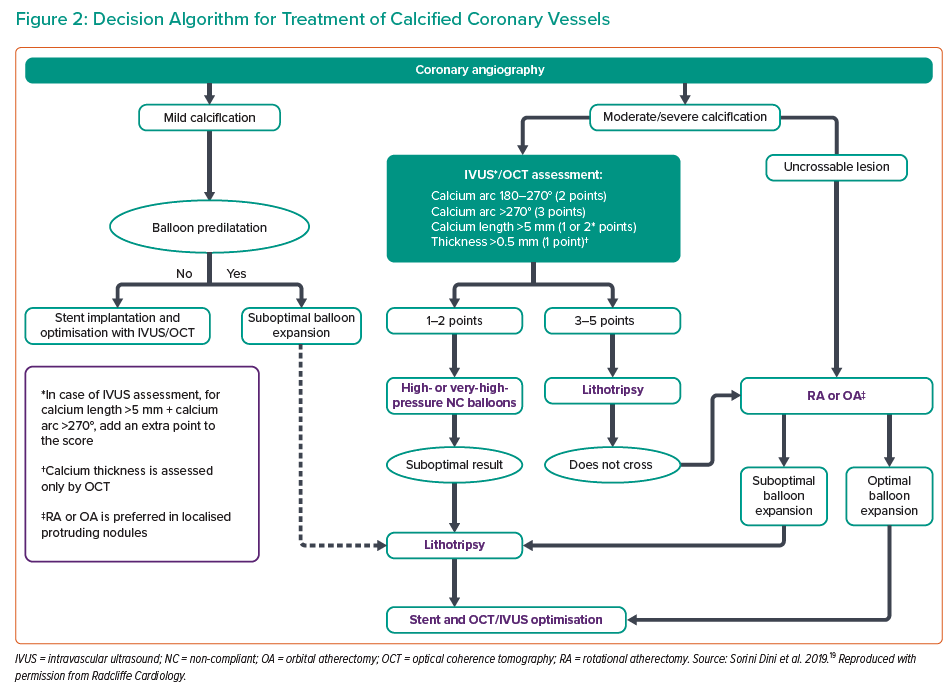
Several studies have suggested a pre-defined algorithm for analysing plaque characteristics and guiding the selection of PCI strategies and plaque preparation using OCT or IVUS (Figure 2).18,19 We consider IVL the therapy of choice in highly calcified lesions defined as having a severe calcium arc (>270°) or calcium thickness >0.5 mm.
Moreover, an interesting finding from the DISRUPT CAD I OCT sub-study is that more extensive calcium modifications, defined by incidence of calcium fracture, calcium fracture per lesion and quadrants of calcium fracture, were achieved in lesions in the highest calcification tertile, suggesting that the higher the degree of calcification, the greater the IVL efficacy.20
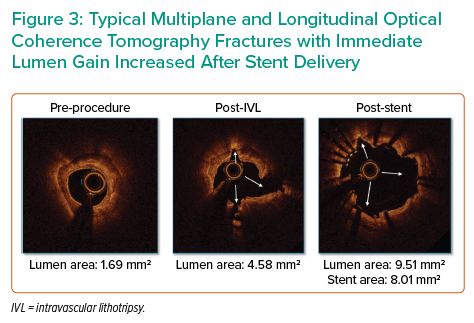
Post-therapeutic Evaluation
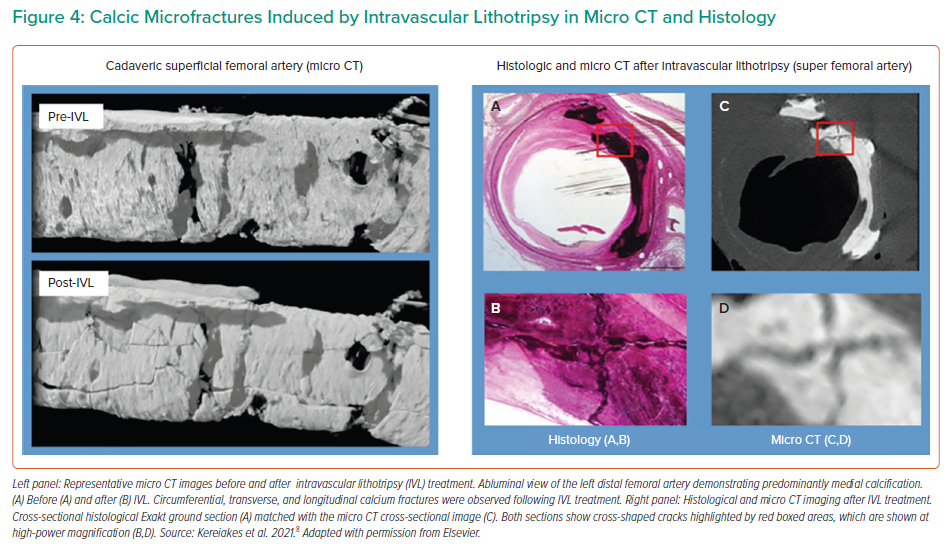
Multiplane and longitudinal fractures are typically observed on OCT after IVL therapy (Figure 3). In a sub-study core lab OCT analysis in DISRUPT CAD III, these fractures resulted in increased vessel compliance with a luminal gain of 1.41 mm2 and 4.35 mm2 at the site of the minimal lumen area after IVL and stent delivery, a mean post-procedural minimal stent area of 6.66 mm2 and full stent expansion defined as mean stent expansion at the maximal calcium site.14 The percentage of lesions with calcium fractures and the maximum calcium fracture depth were similar in post-IVL and post-stent acquisition; however, the maximum fracture width increased after stent expansion (from 0.55 ± 0.45 mm after IVL to 1.32 ± 1.04 mm after stent implantation). Another notable finding was that fracture incidence occurred in 67.7% of patients but without differences in angiographic, OCT or clinical outcomes.14 This suggests that the absence of calcium fracture on OCT is not a sign of failed therapy as the fracture can be ‘out of plane’ and acoustic waves induce calcification microfractures beyond the resolution of OCT technology, as shown on micro-CT and histology in Figure 4.8
Limitation of Endovascular Imaging
Access to intravascular imaging can be limited in different countries and can be because of cost. Moreover, crossability of the probes (IVUS or OCT) through complex calcified lesions can be challenging. Careful analysis of the baseline angiogram before contrast injection is crucial to classify the lesion according to the classification used by Mintz et al. and to predict PCI complexity and IVL success.21
Specific Clinical Situations
Left Main Lesions
The feasibility and safety of IVL in calcified left main (LM) lesions are supported by a retrospective analysis of 31 lesions treated by IVL. In this study, the target minimal stent area was achieved in 97.3% of stented segments with no in-hospital MACE.22
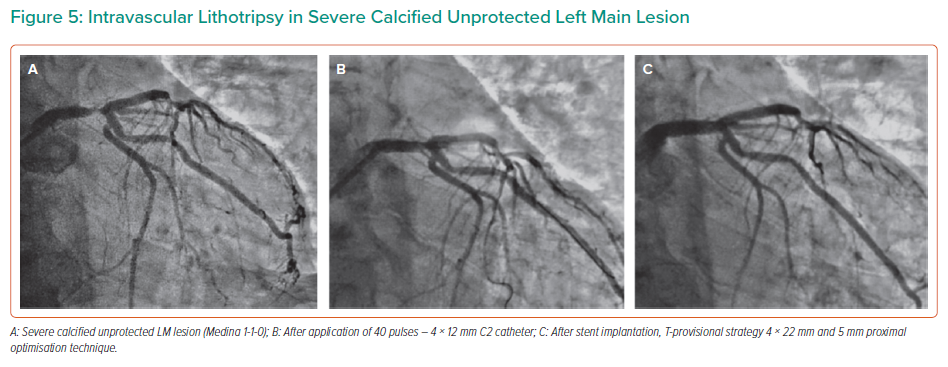
Similarly, good results were provided in a prospective analysis of a registry cohort of 23 patients, in which the primary endpoint (successful stent delivery and expansion with attainment of <30% in-stent residual stenosis of the target lesion in the presence of thrombolysis in MI flow grade 3) was achieved in all patients.23 In this study, the mean IVL catheter diameter used was 3.7 ± 0.3 mm, and a median of eight cycles and 80 pulses were applied (interquartile range: 47–80). In large LM lesions, a 4.0 mm IVL catheter may be used to fracture the calcium, followed by a 1:1 sized NC balloon to expand the fractures created by IVL and prepare for a larger stent (Figure 5).
Among the concerns with IVL for LM lesions is the need for prolonged vessel occlusion to deliver the required energy, which could lead to severe ischaemia.
Salazar et al. distributed the energy by providing pulses individually or in small groups with shorter balloon inflations to minimise this risk. With this strategy, only 2 of 23 patients experienced severe arterial hypotension during inflation.23
Nodular Calcium Lesions
In these lesions, more pulses may be required to modify eccentric calcium as the emitter is further away from the calcium root, and there is none of the wave reflection seen with concentric calcium. If all 80 pulses have been applied, an NC balloon can help determine the procedural efficacy and whether an additional IVL catheter is needed to ensure full balloon expansion.
Long and Multiple Lesions
Ideally, IVL treatment should be applied from distal to proximal lesions. However, in long lesions, the operator can start by advancing the catheter as distally as possible, delivering energy and continuing to advance the catheter. To ensure full therapeutic coverage in the vessel, a 2 mm overlap is advisable when delivering energy in consecutive treatment zones. In case of multiple lesions, we recommend the delivery of 20 pulses for each lesion and subsequently assessing the optimal points for remaining pulses. It needs to be kept in mind that multiple wires within the catheter enable transmission of the energy from the generator to the emitters, which may be damaged during rigorous manipulation.
Difficulty in Crossing the Lesion with IVL
If the electrodes embedded in the balloon are a prowess of technical miniaturisation, the catheter has a low profile, with a bench crossing profile of 0.042" ± 0.002. Every tip and trick used in advanced PCI, such as buddy wire, anchoring and guiding catheter extension, can be applied to overcome deliverability issues. The 6 Fr guiding catheter extensions are compatible with all the diameters of the C2 catheter.
Critical Lesions
In Disrupt CAD II, pre-dilation with a balloon catheter was allowed to ensure crossing of the IVL catheter. This was necessary in 41.7% of cases, using an average balloon size of 2.2 ± 0.6 mm.11 In case of failure to cross, a hybrid rotational atherectomy approach with additional intracoronary lithotripsy (rotatripsy) can be used (as previously described by Jurado-Romàn et al.) to deliver IVL and modify both superficial and deep calcium, especially in large vessels.24
From a technical perspective, it is essential to have sufficient space in the balloon to allow the formation of vapour bubbles to optimise sonic output. However, as the combination of atherectomy and IVL increased the cost of the procedure, this hybrid approach should be reserved in case of failure of one device (IVL or atherectomy bail out) and not as a front-line technique. The analysis of the pre-PCI angiogram is crucial to decide which are the best tools according to local resources and operator experience.
IVL and Stent Underexpansion
Stent underexpansion is a dramatic situation in coronary intervention leading to in-stent restenosis and/or stent thrombosis.25 Although there are numerous clinical cases in the literature reporting the efficacy of IVL in the treatment of restenosis related to underexpanded coronary stent, there are no robust data to support this off-label indication for IVL.26
A study with a smaller cohort by Aksoy and al. suggests that using IVL in an in-stent restenosis (ISR) cohort has a procedural success lower than for de novo lesions.27 Moreover, there are still pending questions regarding the necessity for using an antiproliferative drug after IVL or potential mechanical consequences of the acoustic burst on metallic scaffolding. However, stent underexpansion related to inadequate plaque preparation is a dramatic situation in which IVL could be a game-changer. More clinical data from daily practice and large-scale registry are needed.
Shocktopics and Electrophysiological Disorders
IVL generates mechanical pulses, which may cause atrial or ventricular capture in patients with bradycardia. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. It is essential to understand that no electrical current leaves the IVL catheter. Instead, a small amount of mechanical energy is transferred to the vessel wall when sonic pressure waves are created that have been shown to create a stretch-activated response in the myocardium. In the event of clinically significant haemodynamic effects, you may have to temporarily interrupt the IVL therapy.
Wilson et al. first described IVL-induced ventricular capture, called ‘shocktopics’. In this retrospective analysis, 77.8% of patients underwent IVL-induced ventricular capture with no resulting adverse clinical events.28 The occurrence and significance of shocktopics have been evaluated in Disrupt CAD III.14 IVL-induced capture was noted during IVL in 41.1% of cases. Decreased systolic blood pressure during the IVL procedure was more frequent in patients with IVL-induced capture than those without (40.5% versus 24.5%, p=0.0007). However, the magnitude of the drop in systolic blood pressure was similar between the two groups (p=0.07). IVL-induced capture did not result in sustained ventricular arrhythmias during or immediately after the IVL procedure in any patient and was not associated with adverse events. Multivariable Cox regression analysis identified that a heart rate of ≤60 BPM, male sex, and the total number of IVL pulses delivered were independent predictors of IVL-induced capture.14
However, one case of VF has been related to off-label use of IVL for in-stent restenosis in a right dominant coronary artery (RCA).29 The authors described a ventricular arrhythmia by IVL ventricular ectopy on a T wave. It is reasonable to think that this is the consequence of multiple parameters, including favourable electrophysiological susceptibility, pre-existing ventricular ectopy and ischaemia on dominant RCA being more prone to ventricular arrhythmia. However, it also provides a warning and highlights the need to be aware of such exceptional but potential side-effects.
IVL Versus Other Calcium Modification Technologies
Calcified plaque preparation is crucial for PCI success. Induced-calcium modifications are significantly different from pre-existing technology (NC balloon, high-pressure balloon, modified balloon and atherectomy device) with subsequent advantages. As described above, the efficiency is powered by acoustic burst and not balloon inflation, whereas in NC balloons or modified balloons avoids vessel wall barotraumatic injury and decreases the risk of arterial dissection. IVL does not suffer from wire bias as does atherectomy (and subsequent eccentric plaque guttering) and there is a decreased risk of vascular bed overload related to debris embolisation. IVL technology also affects the deeper calcium, whereas debulking technologies are limited to the superficial calcium, which may negatively affect vessel compliance. Last, in contrast to procedures such as atherectomy, the technology can be easily adopted, not least since it requires no specific training as the device is delivered similarly to standard catheter-based PCI.30
Conclusion
As a novel technology, published data on IVL includes highly selected clinical situations and patients, but are reinforced by operator experience in daily clinical practice. This growing experience and new trials would fill the gaps remaining in the current scientific literature for situations not encountered in the Disrupt studies. It would be interesting to have a comparator to IVL for plaque preparation, such as a modified balloon or atherectomy. In addition, the registry databases with real-world data will continue to grow and provide valuable information, particularly on the safety of IVL. With increasing uptake, we can expect further methodological advances and information from the application of IVL to more complex clinical situations.
IVL is a promising therapy for complex calcified lesions with a short learning curve and a favourable safety profile. However, knowledge of the technical characteristics of the catheter and appropriate considerations in terms of preparation, use and specific conditions for IVL will improve daily results and outcomes in patients presenting with complex calcified coronary disease.