Professor Patrick Hunziker is the Deputy Chief of the Intensive Care Unit of the University Hospital Basel. He obtained his MD from the University of Zurich with post-graduate training at Massachusetts General Hospital and Harvard Medical School. Professor Hunziker has authored more than 140 peer-reviewed articles.
Professor Hunziker began by discussing the need for and limitations of evidence-based medicine. While it is established that the strongest evidence for a therapeutic intervention is obtained from a systematic review of randomised controlled trials involving a homogeneous patient population, each human is unique. There are more potential human phenotypes than atoms in the universe. Similarly, the risk factors for cardiovascular disease are so numerous that it is impossible to produce an algorithm for assessing risk in clinical practice. In addition, acute myocardial infarction (AMI) is a disease that changes over time and is a different disease even after 3 hours. Considering these factors, the conventional requirements of a clinical trial (i.e. a homogeneous patient population) do not exist.
There is a need for knowledge-based, individualised medicine with an emphasis on an interdisciplinary approach. We must focus on and learn from individual patients, employing personalised treatment modalities, and adapt treatment regimens on a case-by-case basis. In AMI, our priority should be the avoidance of death and minimising myocardial necrosis through improved hospital management, the use of percutaneous coronary intervention (PCI) in unstable patients and the management of cardiogenic shock (CS).
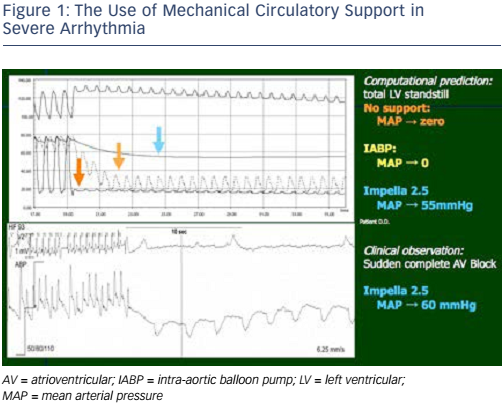
Prof Hunziker has implanted around 300 Impella® (Abiomed) devices and provided practical insights from his experience of using bedside simulations. The first case was of a patient in severe arrhythmia. The mean arterial pressure (MAP) was zero. The use of an intra-aortic balloon pump had no effect on MAP; however, the use of the Impella raised the MAP to 55 mmHg (see Figure 1). The same patient subsequently experienced sudden complete atrioventricular block. By using the Impella 2.5 in this patient, MAP was maintained at 60 mmHg. In the second case, a patient presented with profound left ventricular (LV) failure. Combining mechanical support and vasodilators proved effective in this patient, and had a beneficial effect on oxygen consumption. In both cases, mechanical support by the Impella gave the physician the advantage of restoring MAP (and perfusion pressure), even in the absence of cardiac function.
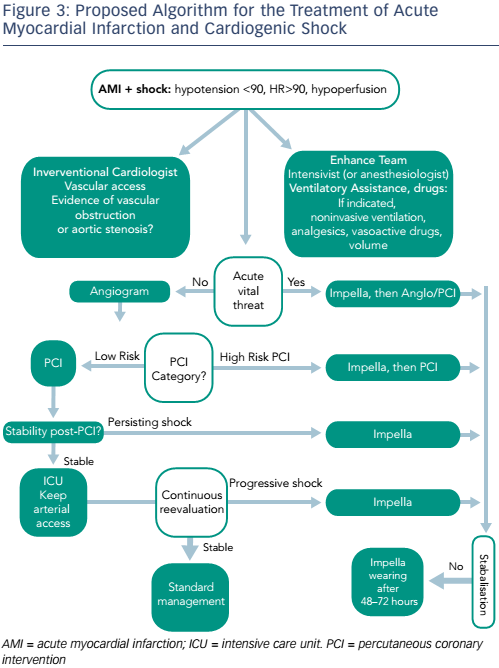
There is a need to optimise the use of mechanical circulatory support (MCS) devices in routine clinical practice. One paradigm currently under investigation is the combined use of MCS and vasodilators in order to optimise organ perfusion in CS, as well as minimising LV wall stress and LV work. A LV assist device (Impella) is employed first, followed by the administration of vasodilator drugs (nitrates or angiotensin-converting-enzyme inhibitors) with a target MAP of 65 mmHg (see Figure 2).
In order to further develop the use of the Impella in CS, there is a need for improved monitoring and a willingness to change approaches based on haemodynamic data. The most important factor in the treatment of CS is time; early haemodynamic support is essential to avoid a systemic inflammatory response. Operator speed is crucial and increases with experience. It is feasible that implantation time may be reduced to 1–2 minutes in the future. However, it is also vital to optimise treatment decisions by correctly incorporating the use of the Impella device within current treatment algorithms.
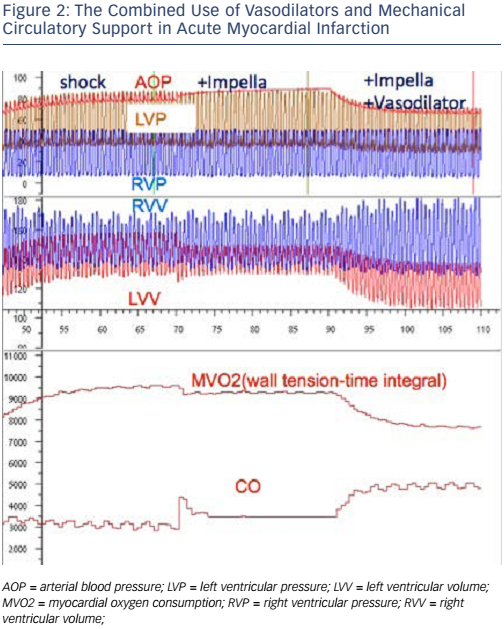
While it may be easy to identify CS, deciding in which patients we should delay reperfusion is less straightforward. Figure 3 shows an algorithm proposed by Prof Hunziker for the treatment of patients presenting with AMI and CS. This algorithm highlights the heterogeneity within both the patient population and the treatment of AMI itself. In selecting patients to undergo Impella implantation, it is useful to consider the potential for brain stem recovery if patients are supported early and given adequate therapies.
This presentation sparked a debate about whether current evidence was sufficient to provide haemodynamic support at small centres. Around 60 % of cases of CS are treated at small centres in the US, with a potential delay caused by patient transportation to a larger centre. In many cases, some form of advanced haemodynamic support might allow these sites to either reperfuse more safely on site and/or facilitate the safer transfer of these patients to expert facilities. The question was raised as to whether all primary PCI centres should be mandated to Impella. The consensus opinion was not at this time but perhaps this will develop over the coming years. Prof Hunziker strongly encouraged centres with primary PCI capability to start MCS on site but to be in close communication with a central hub. There was a recognised lack of sufficient evidence to indicate that MCS be initiated in these centres followed by patient transfer. Problems relating to geography may become an important factor, as journey times to a central hub may be long in some regions.
Prof Hunziker concluded by observing that the A-CURE symposium provides an excellent platform for future progress towards new paradigms in AMI and cardiopulmonary resuscitation. The use of modelling and monitoring will enable the optimal use of new technologies for personalised disease management.