Joseph Hill is a Professor in the Department of Internal Medicine’s Division of Cardiology and the Department of Molecular Biology at UT Southwestern Medical Center and is the Chief of Cardiology and Director of UT Southwestern’s Harry S Moss Heart Center. He is the current Editor-in-Chief of Circulation.
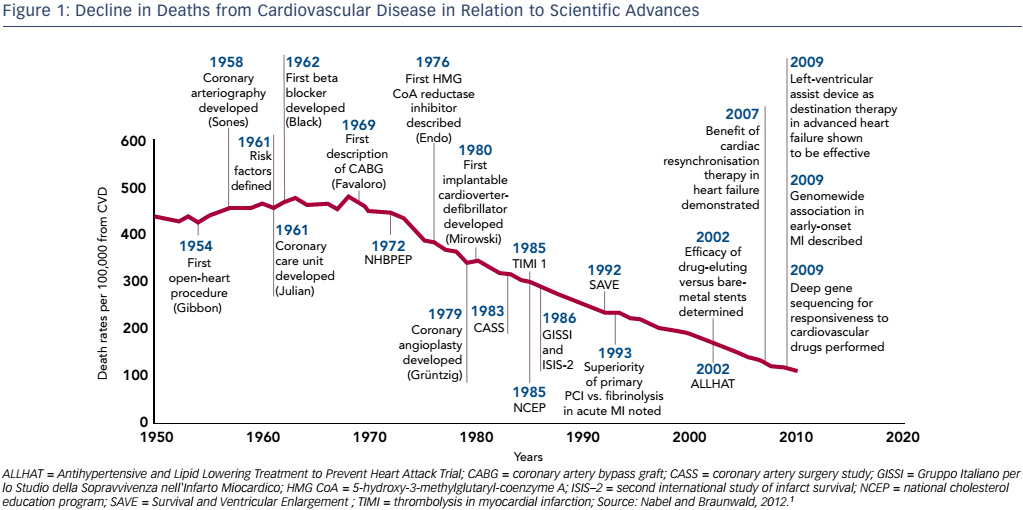
Professor Hill’s presentation placed the concept of myocardial unloading into the broader topic of heart failure (HF), a growing clinical, economic and social problem due to its increasing incidence and poor prognosis. At present we cure very few diseases but instead turn acute disease into chronic disease, which we manage progressively. Thanks to numerous clinical advances in the management of acute myocardial infarction (MI), in-hospital mortality has fallen substantially, from 30 % in the 1960s to around 2–3 % today, and we are moving into an era of lifelong disease management.1 The decreased mortality from cardiovascular disease is impressive when compared to other diseases. The decline in age-adjusted mortality in relation to scientific advances is 75 % (see Figure 1); by comparison, the reduction for cancer is 10 %.1 However, this reduction in MI mortality has been accompanied by an increased incidence of HF2,3 and thus, despite these successes, cardiovascular disease remains the leading cause of death worldwide. There is a need to halt the rise in HF incidence. This has led to an upsurge in interest in mechanical circulatory support devices, which form the focus of the symposium.
A major cause of HF is cardiac remodelling due to hypertrophic growth, the primary mechanism by which the heart reduces stress on the ventricular wall. The heart is a remarkably plastic organ and can grow by up to 50 % under different circumstances including exercise and pregnancy, but also in pathological conditions such as hypertension and infarction.4 These changes can occur rapidly. The heart can also atrophy by up to 70 % in situations such as the use of implantable ventricular assist devices, cancer and bed rest.4 Factors contributing to remodelling include elevated preload, ischaemia, metabolic and neurohumoral factors. One signalling pathway known to be responsible for disease-related plasticity involves class I and II histone deacetylases (HDACs). If a heart is exposed to thoracic aortic constriction to increase afterload, it will grow by about 40 %. If the heart is then exposed to a broad-spectrum inhibitor of HDACs, the growth response is halved, suggesting that some of the growth is HDAC-dependent.5 HDAC inhibitors can also reverse pathological cardiac hypertrophy and restore cardiac function by suppressing autophagy.6
Another example of myocardial plasticity focuses on metabolic stress due to the deterioration of healthy lifestyles.7 Obesity trends in the US over the past 25 years show astounding changes in prevalence. In 1995, all US states had obesity rates >10 %. By 2000, only one state had obesity rates <15 % and rates of 20–24 % emerged. By 2005 we saw the emergence of obesity rates >30 %. By 2010, all states had obesity rates of at least 20–24 %.8 These trends in obesity are now being seen worldwide9 and are accompanied by the rising prevalence of diabetes. It is estimated that 50 % of the Chinese population is prediabetic; consequently diabetes-associated heart disease is set to become a global pandemic. Expert cardiologist Eugene Braunwald has stated that: “The thrombcardiologist of the 20th century will be replaced by the diabetocardiologist of the 21st century.”
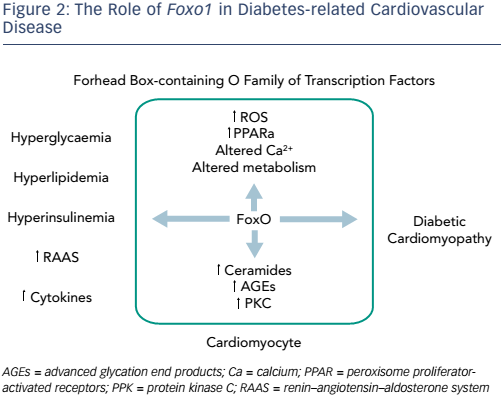
Recent advances in understanding of the pathophysiology of diabetes have identified potential new therapeutic targets. The forkhead boxcontaining O family of transcription factors (FoxOs) regulate essential cellular functions and are emerging as key mediators in cardiac insulin signalling and myocardial plasticity.10 Patients with diabetes have atherosclerotic disease, hypertension and a toxic intra-myocyte milieu. FoxOs are capable of rendering a cell insulin-resistant in vitro11 and have been found to be activated in the cardiac tissue of mice with diabetes. FoxO activity is linked with many aspects of myocardial plasticity, which may be reversed by the deletion of Foxo1. Activity includes cardiac dysfunction and cardiac remodelling, glucose dysregulation, a shift in substrate utilisation and lipid accumulation, as well as metabolic stress-induced ventricular dysfunction, structural remodelling and cardiac fibrosis.10 Administration of tamoxifen to animals exposed to a high-fat diet silences Foxo1 and allows the heart to undergo robust recovery (unpublished data). The ability to metabolise glucose can also be attenuated with tamoxifen.
Another growing public health problem is HF with preserved ejection fraction (HFpEF). HFpEF occurs in 40–60 % of newly-diagnosed HF cases, has an annual mortality of 3–30 % and accounts for a healthcare expenditure of more than $20 billion in 2010.10 In contrast to HF with a reduced ejection fraction, patients with HFpEF still do not benefit from evidence-based treatment options. It remains one of the most challenging clinical syndromes because there are no reliable preclinical models and so it is impossible to develop new therapies. None of the currently-available therapies have shown improved clinical outcomes in trials of HFpEF and these patients’ prognosis has remained unchanged over the past 15 years.11 This is partly due to the numerous proposed pathophysiological mechanisms underlying the condition, which involve multiple organs.12 If current trends persist, HFpEF will spread into the developing world and be responsible for 7.8 million premature cardiovascular disease (CVD) deaths in 2025.13 In addition to this, the costs of CVD, both direct and indirect, are projected to increase substantially.14
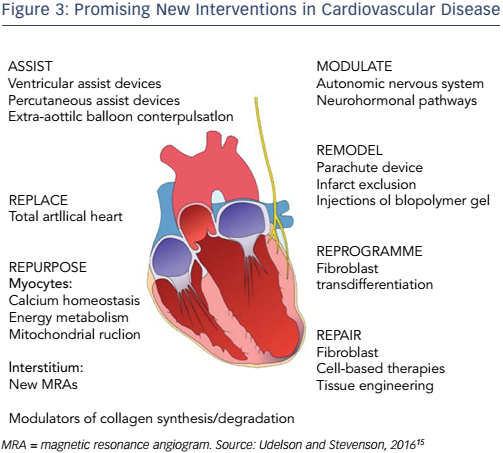
Despite the challenge ahead, Professor Hill ended on a note of optimism. A number of new interventions for CVD appear promising (see Figure 3).15 Projections have shown that National Institutes of Health funding translates into improvements in CVD mortality.16 Furthermore, opportunities lie in the field of genetics. It has been hypothesised that cardiomyocyte-specific Foxo deletion will sustain cardiac function in the setting of insulin resistance.17 By focusing on the molecular basis of diabetes, we have the potential to mitigate the projected healthcare cost of this growing epidemic. Finally, as highlighted by other presentations at the A-CURE symposium, mechanical unloading can minimise the infarct size and thus has the potential to reduce the incidence of HF.