Kenji Sunagawa is the founder and Professor of the Center for Disruptive Cardiovascular Medicine at Kyushu University, Fukuoka, Japan. He joined the cardiovascular group at the Department of Biomedical Engineering, Johns Hopkins University, in 1978 and helped establish the concept of the pressure–volume relationship of the heart.
Dr Sunagawa began by highlighting the concept of ischaemia as an imbalance between oxygen supply and demand. Previous interventions have focused on increasing oxygen supply, but this approach may be insufficient to improve outcomes. Mechanical unloading has the effect of decreasing oxygen demand and can be considered ‘functional reperfusion’. However, while it is possible to dramatically reduce myocardial oxygen consumption by the use of left ventricular assist devices (LVADs), Dr Sunagawa is exploring additional complementary approaches to further reduce myocardial oxygen demand to provide cardiac protection.
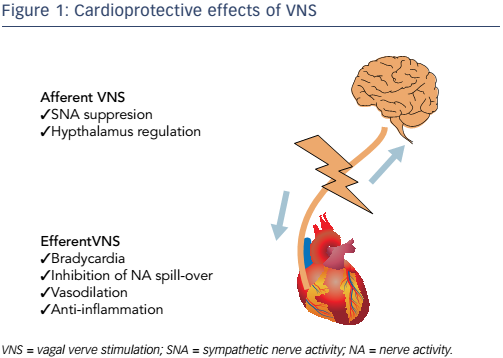
Another approach to myocardial protection is vagal nerve stimulation (VNS). It is well established that VNS has anti-ischaemic effects. These are mediated by complex mechanisms, including heart rate reduction, anti-adrenergic effects and anti-inflammatory effects (see Figure 1).1 Various preclinical studies have demonstrated that VNS results in marked reductions in infarct size following acute myocardial infarction.1–6 Since VNS removes acute myocardial infarction-induced neuromechanical and inflammatory stress, it has been termed neural unloading.
This technique of neural unloading has recently been investigated in conjunction with mechanical unloading. In order to stimulate the vagal nerve in a minimally invasive manner, a catheter with electrodes was inserted into the superior vena cava of a dog. This rapidly decreased the heart rate and, as a consequence, reduced myocardial oxygen consumption by >50%.7
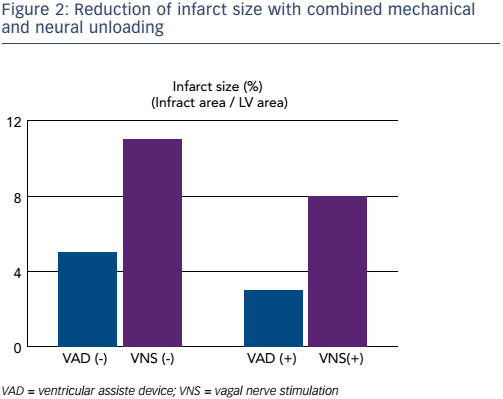
In order to investigate the impact of this investigation on infarct size, an ischaemia reperfusion model was created in 24 dogs by occluding the left anterior descending coronary artery for 180 minutes and then reperfusing. Left ventricular unloading was performed with the Impella CP® (Abiomed) device. Transvascular VNS was performed with a pacing catheter in the superior vena cava. The neuromechanical unloading started 90 minutes after the onset of ischaemia and ended 60 minutes after reperfusion. Dogs were then assigned to one of four groups: ischaemia reperfusion (n=7), LVAD (n=6), VNS (n=4) and LVAD plus VNS (n=5). One month after the intervention, the infarct size and cardiac function were compared. The use of LVAD plus VNS reduced the infarct size by >70% (p<0.05; see Figure 2).8
This combined strategy of mechanical and neural unloading is clearly a powerful intervention and provides almost complete ventricular support. A key factor underlying the success of this approach is the heart rate reduction provided by the combined technique. It has therefore been hypothesised that the combination of mechanical circulatory support and pharmacological heart rate reduction may be beneficial in acute myocardial infarction. The If inhibitor ivabradine is a bradycardic agent; ongoing studies are investigating the combination of ivabradine and LVADs on oxygen consumption in acute myocardial infarction.9
Dr Sunagwa concluded that the combination of LVAD and VNS synergistically reduces infarct size beyond that observed by LVAD unloading alone, preserves left ventricular function, and prevents heart failure in the long term. In order to establish total unloading as a treatment for acute myocardial infarction in humans, the development of higher flow percutaneous pumps is essential. In addition to this, in order to maximise the beneficial impacts, the simultaneous regulation of LVAD and VNS needs to be optimised in further studies.