Transcatheter aortic valve replacement (TAVR) has become a well-established treatment option for patients with severe aortic stenosis (AS), supported by a large body of scientific evidence demonstrating the efficacy and safety of this approach compared with surgical aortic valve replacement (SAVR).1–5 Real-world data has demonstrated comparable clinical outcomes with TAVR to those seen in randomised trials, suggesting that this technology can be successfully incorporated into daily practice outside major academic centres. As a result, TAVR programmes have emerged in most countries outside Europe and North America, including the Middle East/Gulf region.6–8
Many nascent TAVR programmes in the UAE and the region tend to be low-volume rather than large, referral-based programmes, perhaps due to the fragmented delivery of advanced cardiovascular care in this region. In light of limited data on TAVR outcomes from the region, it remains unknown whether procedural outcomes at lower-volume centres with less expertise will be comparable to those achieved at high-volume programmes in developed countries, particularly since an inverse relationship has been shown between TAVR procedural volume and clinical outcomes.9,10 Describing patient characteristics and procedural outcomes at low-volume TAVR programmes will provide insight into the effectiveness of TAVR in the region and will highlight procedural and patient selection considerations necessary for safe and successful real-world adoption of this technology, especially as TAVR use extends to low-risk patients.
It is in this context that we sought to evaluate baseline characteristics and procedural outcomes of patients undergoing TAVR at a newly established programme in the United Arab Emirates (UAE). Lessons learned from this experience may provide a framework for other programmes to achieve desirable patient outcomes, despite not meeting the procedural volume and other requirements described in consensus documents.11
Methods
Study Site and Patient Population
This study prospectively evaluated consecutive patients with severe AS who underwent transfemoral TAVR at a single centre in Abu Dhabi in the UAE between January 2016 and November 2021. The study location is a 393-bed quaternary care hospital that cares for patients with a range of cardiovascular pathologies. The indication and eligibility for TAVR were determined by a multidisciplinary heart team, carefully considering the patient’s age, operative risk, comorbidities and anatomical risk. Functional and cognitive ability and life expectancy were taken into consideration when deciding whether aortic valve replacement would lead to significant and meaningful clinical improvement. For patients with low operative risk as determined by the Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score, inclusion and exclusion criteria specified in the low-risk TAVR trials had to be met.4,5 Patients undergoing TAVR via transapical or transaortic access routes were excluded, as were patients undergoing salvage procedures to reduce potential confounding. All STS-PROM risk groups were included in the study, including those considered to have a prohibitive risk for surgery or were deemed inoperable. This study was approved by the Institutional Review Board and Research Ethics Committee.
Study Variables
Baseline characteristics, including demographics, comorbidities, STS-PROM risk score and echocardiographic variables were systematically collected during patient evaluation for procedures. Clinical endpoints at 30 days were evaluated using Valve Academic Research Consortium (VARC)-2 definitions and included the following: all-cause mortality, stroke, bleeding complications, vascular complications, conduction disturbances, arrhythmias and paravalvular regurgitation (PVR).12 Grading of PVR involved quantitative and qualitative parameters endorsed by the American Society of Echocardiography and VARC-2 guidelines.12,13
Procedural Details
Initially, all procedures were performed in a hybrid operating room under general anaesthesia. As the TAVR programme continued to mature, a ‘minimalist approach’ was adopted for most patients where TAVR procedures were performed under conscious sedation or monitored anaesthesia care, without the need for transoesophageal echocardiography or invasive haemodynamic monitoring and using single femoral artery access, with the aim of facilitating post-procedure recovery and achieving early discharge within 24 to 48 hours if set clinical criteria were met.14–16 Third-generation balloon-expandable valves (SAPIEN 3, Edwards Lifesciences) and self-expanding valves (Evolut-R PRO, Medtronic) were used exclusively during the study period.
Statistical Analysis
Baseline and procedural characteristics for patients undergoing TAVR at the study centre were reported as mean ± SD (or median and interquartile range [IQR] for variables that did not have a normal distribution) for continuous variables and as counts/percentages for categorical variables. To aid interpretation of the results, we compared procedural outcomes at the study site with hospitals in the US with the lowest annualised number of TAVR procedures as recorded in the first quartile in the TVT registry data.9 These hospitals (n=140) will be referred to as Q1 hospitals with 5–36 transfemoral TAVR procedures performed per year. Published summary-level data on patient characteristics and unadjusted rates of clinical outcomes, including 30-day all-cause mortality, stroke, vascular access and bleeding complications, were used as published by the study investigators in the main manuscript or in supplemental material as access to individual patient data was not available.9 All analyses were performed using JMP version 16.
Results
Patient and Hospital Characteristics
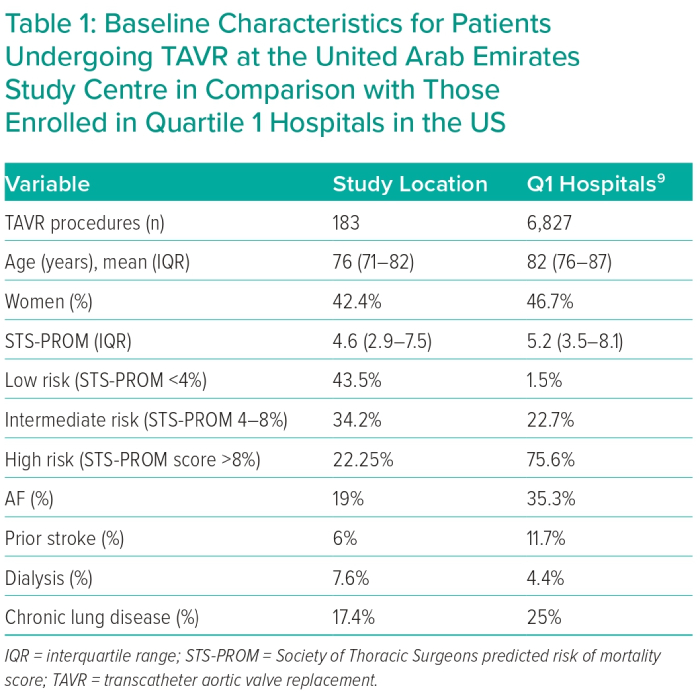
A total of 189 patients underwent TAVR during the study period with an average of 34.5 procedures each year. Six patients were excluded from this analysis since they underwent transapical or transaortic access (n=4) or salvage procedures (n=2). The number of TAVR procedures increased over time from 15 procedures in 2016 to 56 procedures in 2021 (p<0.001). Patients who underwent TAVR in this study were younger compared with those at Q1 hospitals in the TVT registry (median age = 76 years IQR 71–82 and 82 IQR 76–87, respectively) with fewer women (42.1% versus 46.7%). The median STS score for included patients was 4.6 (IQR 2.9–7.5), compared with 5.2 (IQR 3.5–8.1) in the Q1 hospitals (Table 1). Most patients (43.7%) included in this study were low risk according to STS score (defined as STS score <4), while 33.9% were intermediate risk (STS score 4–8) and 22.4% were considered high risk (STS score >8). Only 1.5% of patients who underwent TAVR at Q1 hospitals were in the low-risk group, compared with 22.7% and 75.6% who were considered intermediate- or high-risk, respectively. Compared with those at Q1 hospitals, patients treated at the UAE centre had a lower prevalence of prior stroke (6% versus 11.7%), AF (19.1% versus 35.3%), and chronic pulmonary disease (16.9% versus 25%), whereas they had a greater prevalence of dialysis (7.7% versus 4.4%). Otherwise, most patients treated in our centre presented with congestive heart failure (86.4%), had a history of coronary artery disease (63.9%), with high prevalence of diabetes and chronic kidney disease (68.3% and 42.1%, respectively).
The mean aortic valve area for included patients was 0.7 ± 0.2 cm2 with a mean baseline aortic valve gradient of 43.2 ± 14.2 mmHg and mean left ventricular ejection fraction of 55.1 ± 14%. Corresponding data from Q1 hospitals were not available.
Procedural Success
Transfemoral access was performed in all patients and 71.6% received monitored anaesthesia while the rest received general anaesthesia. Balloon-expandable SAPIEN 3 valves were used in most patients (93.3%), with a median valve size of 23 mm (IQR 23–26). Device success was achieved in 99.5% of patients, while two patients (1.1%) required implantation of a second valve due to device embolisation. One patient (0.5%) experienced annular rupture, while no patients required conversion to surgery or experienced acute coronary obstruction.
Clinical Outcomes
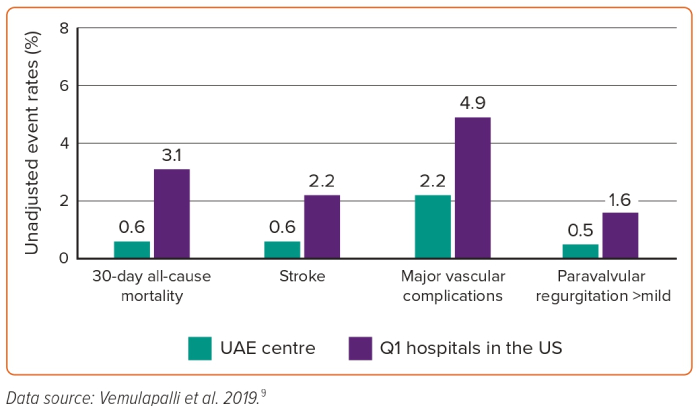
All-cause mortality within 30 days of TAVR procedure was observed in 0.6% of patients treated at the study site, compared with 3.1% in Q1 hospitals. Similarly, stroke rates were lower in this study at 0.6% compared with 2.2% in Q1 hospitals. Major vascular complications occurred in 2.2% and 4% of patients treated locally and at Q1 hospitals, respectively. VARC- major bleeding occurred in 2.2% and 4.9%, respectively. Significant paravalvular regurgitation (more than mild) occurred in 0.5% of patients at the study site, compared with 1.6% in those treated at Q1 hospitals (Figure 1). Pacemaker implantation post-TAVR was needed in 2.7% of patients at the study location and corresponding data from Q1 hospitals were not available.
Discussion
The current study reports characteristics and outcomes of consecutive patients undergoing transfemoral TAVR at a single centre in UAE, and shows favourable 30-day outcomes compared to those seen at hospitals participating in the TVT registry with comparable TAVR procedural volumes.9 It has been shown that patients undergoing TAVR at the study location were younger, with a lower calculated STS-PROM risk score, reflecting the growing use of TAVR in patients with low operative risk. The current findings add to the available literature on TAVR usage and outcomes in the Gulf region and underscore that emerging valve centres in the region can achieve comparable results by conducting meticulous patient selection and the adoption of best practices to optimise procedural success and outcomes.
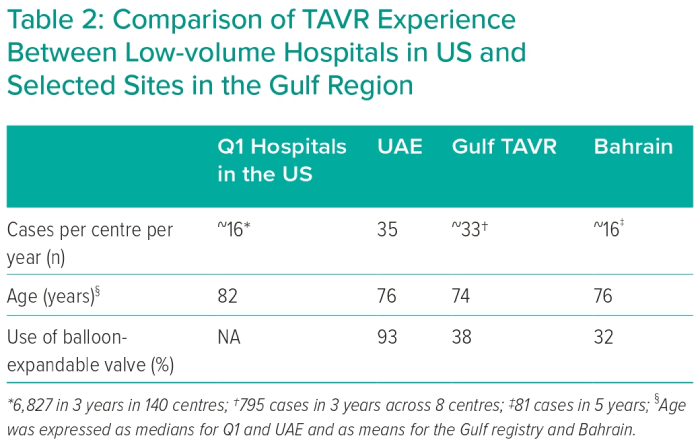
These data also help contrast this single-centre experience from the UAE with published data elsewhere in the Gulf region (Table 2).6,7 Overall, annualised procedure volumes (per centre) and the average age of included patients are comparable across sites. Furthermore, patients undergoing TAVR in the Gulf region were generally younger than their counterparts treated at Q1 hospitals in the US, perhaps due to the difference in life expectancy between the two regions.17 The use of balloon-expandable valves varied across sites, with 93% use in the current study from UAE versus 32–38% in other countries in the region. This may reflect differences in operator training or access to technology at different sites. Unfortunately, variation in endpoint definition across included studies precludes meaningful comparison of clinical outcomes among sites in the Gulf region.
Several aspects of the current experience of establishing a TAVR programme in the Gulf region are worth mentioning. First, patients were evaluated for TAVR eligibility not only based on anatomical and functional inclusion criteria as described in major landmark clinical trials but also based on thorough assessment of frailty and life expectancy, using widely accepted approaches. It was important that low-risk patients passed all exclusion criteria defined in the low-risk TAVR trials. Potential candidates were evaluated for eligibility by a dedicated multidisciplinary heart team, including interventional cardiologists with expertise in structural heart interventions, cardiac surgeons, multi-modality cardiac imagers and a cardiovascular anaesthesiologist.
Second, operators adopted a methodical approach to minimise post-procedural complications. This included comprehensive assessment for paravalvular regurgitation, using echocardiography, invasive haemodynamics and aortography in all patients, with liberal post-dilation of transcatheter heart valves as needed to minimise or eliminate paravalvular regurgitation.18–20 Furthermore, understanding the importance of pacemaker implantation, a ‘high implantation technique’ was adopted early on to reduce the need for pacemaker implantation post-TAVR.21 This technique involves positioning the valve according to the non-coronary cusp (usually the deepest of the sinuses) in the cusp-overlap view (right anterior oblique/caudal projection) with use of fluoroscopy guidance to optimise any parallax from the valve and identification of a coplanar view using pre-procedural multidetector CT. Afterwards, the valve is positioned based on the superior aspect of the most proximal set of stent struts, seen as a radiolucent line on a crimped SAPIEN 3 valve. Last, a ‘minimalist approach’ to TAVR was also adopted early on, which minimised post-procedural morbidity and expedited early patient dismissal.14
Study Limitations
The major limitations of our study include the fact that it is a single-centre experience with site-reported outcomes that were not independently adjudicated by a core lab. In addition, this is a retrospective, observational analysis with limited in-hospital and 30-day follow-up. Patient-level data were not available for analysis from the TVT Registry or from the study by Vemulapalli et al. evaluating procedural volume and outcomes and we relied on available data from published reports.9
Conclusion
In this analysis of TAVR outcomes in the UAE, favourable procedural and clinical outcomes were achieved despite overall low procedural volume. Excellence in transcatheter interventions requires a well-functioning multidisciplinary heart team, careful patient selection and technical expertise. Careful patient selection is critical and may help optimise patient outcomes, especially when procedural volumes are low. 
Clinical Perspective
- Despite low volumes, newly established TAVR programmes may still achieve outcomes comparable to those at more established centres.
- Systematic evaluation of patients and multidisciplinary pre-procedural assessment help to anticipate and prevent complications.
- Establishing regional valvular heart disease centres of excellence helps advance transcatheter interventions in the region.










