Following demonstration of the first percutaneous transluminal coronary angioplasty in the 1970s (and subsequently the early use of pressure wires in the 1990s), an epicardial stenosis-centric treatment strategy has been widely adopted.1–3 However, recent landmark trials, such as the Initial Invasive or Conservative Strategy for Stable Coronary Disease (ISCHEMIA) and Percutaneous Coronary Intervention in Stable Angina (ORBITA), question this approach and refocus attention on the critical role the coronary microcirculation plays in ischaemic heart disease.4,5
Epicardial vessels exist mainly to perform a capacitance function, exerting minimal resistance to coronary blood flow.6 In contrast, the microcirculation (comprising of pre-arterioles, arterioles and capillaries) plays the main role in the autoregulation of myocardial blood flow, potentially facilitating a fivefold increase in coronary flow to meet increased myocardial oxygen demands.7
Coronary microcirculation dysfunction (CMD) represents an area of unmet therapeutic need and a diagnostic challenge, with the potential to manifest in both acute and chronic coronary syndrome (CCS), be that in the presence, or absence, of epicardial coronary disease.8 In CCS, up to one-third of patients will continue to experience angina following percutaneous coronary intervention (PCI).9 Indeed, there is growing evidence suggesting that CMD contributes to persistent angina in patients with CCS without significant coronary obstruction.10 The recent Coronary Microvascular Angina (CorMicA) trial was a randomised controlled trial that showed significant symptomatic benefit to patients randomised to invasive diagnostic assessment of microvascular function with subsequent stratification of medical therapy, resulting in a European Society of Cardiology Class 2a recommendation for the invasive assessment of microvascular function in patients with ischaemic anginal symptoms with non-obstructive coronary arteries (i.e. ischaemia with non-obstructive coronary arteries [INOCA]).11,12 In ST-elevation MI (STEMI), CMD is present in as many as 50% of patients despite timely reperfusion and is associated with adverse clinical outcomes, such as all-cause mortality and hospitalisation with heart failure within 1 year, as well as early major cardiac complications.13–16 Furthermore, abnormal microvascular resistance after primary PCI has been shown to predict infarct size, left ventricular ejection fraction and myocardial salvage.17
Animal models show there is a narrow time window after reperfusion in which potential therapies could be initiated, emphasising the need for timely diagnosis of CMD.18 Accordingly, the routine upfront quantification of microvascular dysfunction at the time of cardiac catheterisation has the potential to deliver a wealth of information that could aid in the risk stratification of patients, resulting in the initiation of tailored treatments and providing potential symptomatic and prognostic improvements across multiple disease groups.
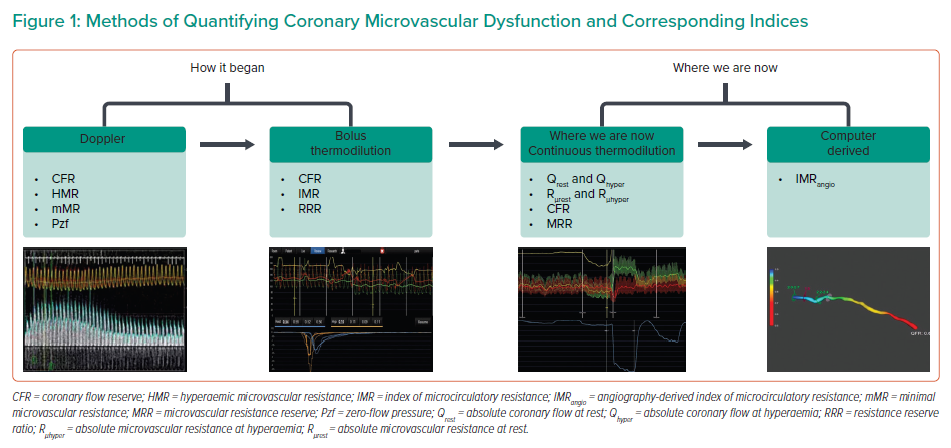
The purpose of this paper is to provide a contemporary review of the methodology underpinning the diagnosis of microvascular dysfunction and to uncover gaps in knowledge. The equipment used in the derivation of microvascular indices will be revisited in addition to practicable indices that have arisen from these methodologies (Figure 1).
Methods of Quantifying Microvascular Function
A healthy coronary microvascular system has the ability to adapt microvascular resistance in order to modulate coronary flow and thus maintain adequate myocardial oxygen supply. Because resistance cannot be directly measured, for clinical purposes it is derived according to the principle of Ohm’s law, namely R = V/I, where R is resistance, V is distal coronary pressure (Pd) and I is coronary flow.
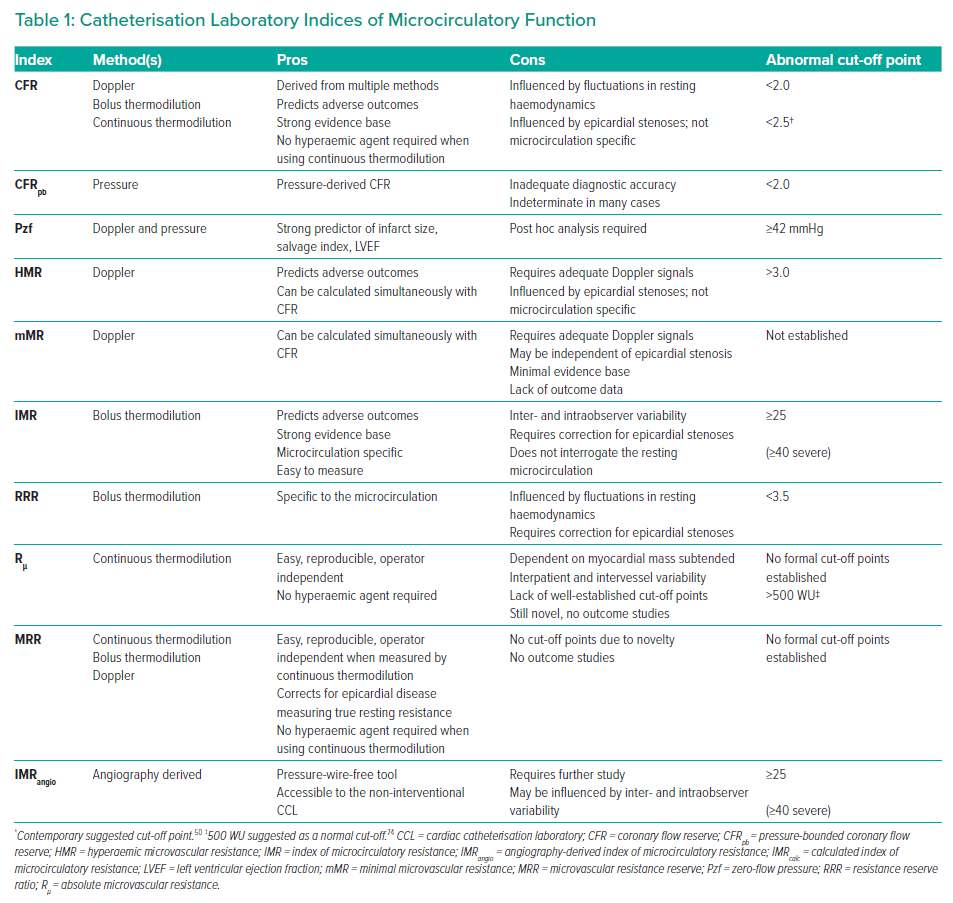
In contemporary practice, coronary flow can be quantified in the cardiac catheterisation laboratory (CCL) using three main methods: combined Doppler/pressure-tipped coronary guidewires; thermodilution using combined thermistor/pressure-tipped coronary guidewires; and, most recently, angiography-derived computation of coronary flow and pressure.19–22 In addition, two methods of thermodilution are described in the literature (bolus and continuous).21,23 Several indices of microcirculatory function have been developed using these methodologies (Table 1).
Doppler/Pressure Wire
Early on in the invasive assessment of myocardial ischaemia, efforts were made to quantify coronary flow velocity using specialised coronary Doppler catheters. However, these were bulky and were not able to subselect the distal coronary arteries.24 Furthermore, even when 3-Fr Doppler catheters (capable of subselecting coronary arteries) were used, the size of the catheters caused significant impedance of antegrade coronary flow, therefore affecting Doppler waveforms.25
After the successful miniaturisation of coronary pressure wires, the intracoronary Doppler wire was developed and the first in vitro and in vivo experiments were performed.26 The wire was trialled in anaesthetised and surgically instrumented canines, in which it was used to compare average peak velocity (APV) derived by Doppler against blood flow measured directly by electromagnetic flow probes.26 These studies found a linear relationship between APV and blood flow measured using electromagnetic flow probes (r2 = 0.86–0.90).26
The Doppler wire was subsequently validated in human studies; however, at the time, the focus was centred on their application in the assessment of coronary lesion severity.27,28
Thermistor/Pressure Wire
The advent of the combined thermistor/pressure coronary guidewire has resulted in two methods of coronary physiological assessment, bolus and continuous thermodilution, which are described below.
Bolus Thermodilution
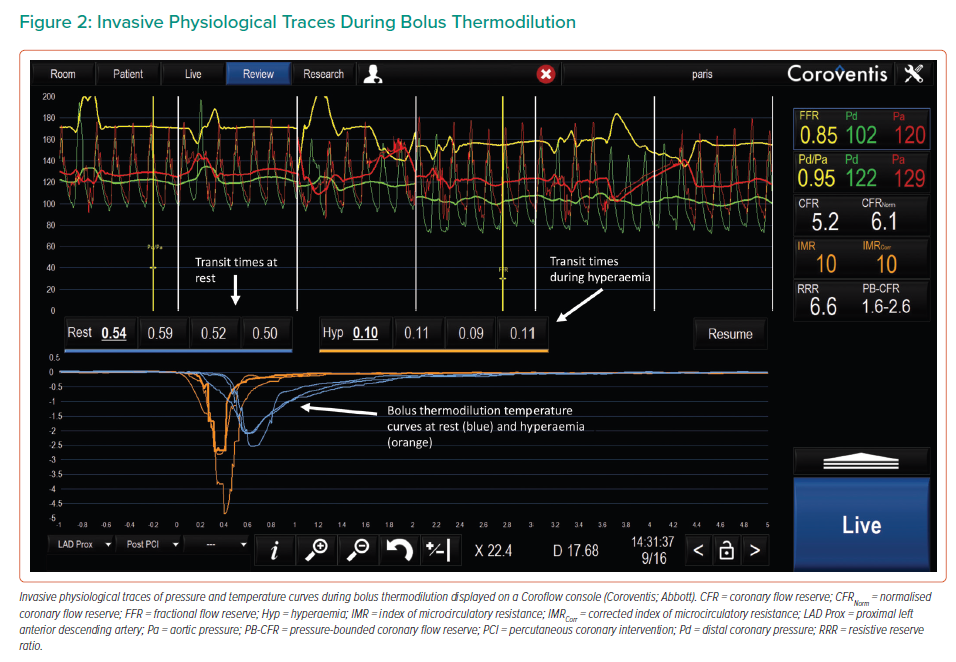
In 2001, De Bruyne et al. first described a method of using a thermistor/pressure wire placed distally in the vessel, in conjunction with a bolus (3 ml) injection of saline, to derive a surrogate for coronary flow.20 This method relies on the indicator dilution theory:
F = V/Tmn
where F is flow, V is the vascular volume between the injection site and thermistor and Tmn is the mean transit time of the saline bolus (Figure 2). Due to the administration of intracoronary nitroglycerin, the vascular volume can be assumed to be constant, resulting in inverse proportionality between flow, F, and Tmn (Figure 2):
F = 1/Tmn
This method was trialled in vitro using flow circuits, where the inverse of Tmn was found to be very closely correlated with absolute flow provided by a pulsatile pump (r >0.95).20 The method was also shown to correlate well with Doppler-derived flow in canines (r = 0.76) and later in human validation studies (r = 0.80).20,21
Continuous Thermodilution
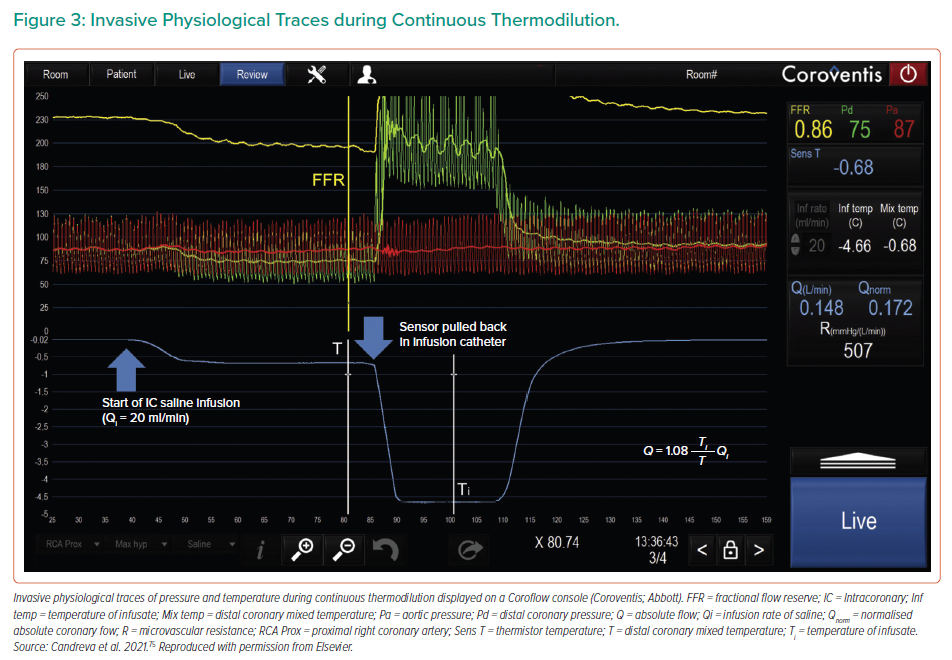
In 2007, Aarnoudse et al. first described a novel method of directly measuring volumetric blood flow in coronary arteries using the principle of continuous thermodilution.23 Using saline infused through a 2.8-Fr infusion catheter in conjunction with a standard pressure/thermistor-tipped guidewire, absolute coronary blood flow (Q) can be calculated using the following equation:
Q = Qi(Ti/T) × 1.08
where Qi is the infusion rate of saline, Ti is the temperature of the infusate at the tip of the infusion catheter, T is the temperature of the mixed blood and saline in the distal coronary and 1.08 is the correction factor used to compensate for the specific heat difference between blood and saline (Figure 3).23
Absolute coronary blood flow, Q, corresponded well with true flow measured in canines using surgically implanted flowmeters (R2 = 0.72), and with changes in fractional flow reserve (FFR) before and after PCI in humans (R2 = 0.84).23 The study also showed excellent reproducibility, including when the guidewire sensor was moved to different positions between 3 and 6 cm away from the infusion catheter. Distances <3 cm from the catheter were not used to avoid incomplete mixing of blood and saline.
The first safety, feasibility and reproducibility study of this method was conducted in humans by Xaplanteris et al. using a dedicated monorail multihole catheter (Rayflow; Hexacath).29,30 A key development in the design of this catheter was the induction of hyperaemia when saline was infused at rates of ≥15 ml/min, thought to be mediated by epicardial vessel wall vibrations and, more recently, haemolysis.31,32 In their study, Xaplanteris et al. used the principles of continuous thermodilution to derive Q and the principles of Ohm’s law to calculate microvascular resistance (µ).29 The method was validated in 135 patients undergoing coronary angiography, showing continuous thermodilution-derived measurements of Q and µ to be reproducible and safe in humans. In addition, this method is quantitative, independent of operators and easy to perform.
A further study by Keulards et al. confirmed the safety of this method.33 On their own, µ and Q are lacking in diagnostic utility due to their dependence on myocardial mass, and therefore exhibit intervessel and interpatient variability.34 They are therefore more likely to find clinical utility when used in microvascular indices.35,36
Continuous thermodilution has opened the door to new research opportunities investigating coronary flow and µ in a more accurate and reproducible way than prior methods.
Computational Software
In recent years, novel angiography-derived methods of assessing FFR have been developed that use computational methods of deriving a flow ratio from diagnostic coronary angiography alone.37–40 These tools have shown promising results in their primary use case as a measure of epicardial stenosis severity; however, their use has recently been expanded to the quantification of microvascular dysfunction in small proof-of-concept studies.41,42
Because the computed flow ratio is intended to be equal to FFR, it can be used to derive Pd by multiplying it by the invasive aortic pressure (Pa) measured during coronary angiography.43 Ohm’s law can then be used to derive a resistance index, using computer-derived coronary flow, or the transit time of contrast on angiography as a surrogate for coronary blood flow.22,41,42
Indices of Microvascular Function
Coronary Flow Reserve
In 1974, Gould et al. published early work that quantified the effects of stenoses of varying severity on coronary flow and myocardial blood flow distribution, conducting experiments on living canines with surgically implanted coronary flowmeters and vessel constrictive devices.44,45 From these experiments, the concept of coronary flow reserve (CFR) was initially developed as a measure of epicardial stenosis severity, where CFR is defined as the ratio of coronary flow during hyperaemia to coronary flow at rest:2

In the years that followed, the validity of CFR was confirmed using non-invasive imaging, Doppler-tipped catheters and thermodilution-derived flow in the coronary sinus, among other methods.2 Although the original intention of CFR was to aid in the quantification of stenosis severity, the fact that it relies on vasodilator-mediated augmentation of coronary flow results in the simultaneous assessment of the microvascular system.2,46–48 The cut-off point for a normal CFR is 2, but most patients with normal coronary arteries and microvascular function will have levels between 3 and 5.11,49 Contemporary literature challenges this cut-off point, suggesting an alternative cut-off value of 2.5.50 A recently published meta-analysis found that reduced CFR was strongly associated with all-cause mortality and major adverse cardiac events (MACE) across a wide range of pathologies and patient groups.51
In practice, CFR can be calculated using surrogates for coronary blood flow and can therefore be derived by:
- using Doppler-tipped coronary guidewires (CFRDoppl), taking APV as a surrogate for coronary blood flow;
- performing bolus thermodilution in conjunction with a combined pressure/thermistor coronary guidewire (CFRbolus), using the inverse of the mean transit times of a bolus of saline at rest and hyperaemia as a surrogate for flow; or
- using a novel method that has recently been demonstrated based on the principles of continuous thermodilution (CFRthermo), which derives absolute coronary flow at rest (Qrest) and hyperaemia (Qhyper), from which CFR can be calculated.19–20,36
Compared with [15O]-H2O PET as a gold standard, CFRDoppl has a better agreement than CFRbolus and exhibits less intraobserver variability.52,53 Nevertheless, the accuracy of CFRDoppl is affected by the difficulty in achieving adequate Doppler signals.53 Although still a method in its infancy, one small study demonstrated that continuous thermodilution derived indices correlate well with doppler derived (r=0.88).35 The CFRthermo method shows promise due to its ease, safety, reproducibility and non-reliance on pharmacologically mediated hyperemia.29,33
Overall, CFR has three main limitations as a measure of microvascular dysfunction. First, because CFR is affected by both the epicardial and microvascular coronary arteries, a reduced CFR is most useful as a direct measure of microvascular dysfunction when there is an absence of physiologically significant epicardial disease.54 Second, because CFR requires measurements in the resting state, it is influenced by fluctuations in blood pressure, left ventricular contractility and heart rate.55 Third, CFR can be low in the presence of high resting flow in the context of revascularised STEMI, and therefore exhibit discordance with other indices of microcirculatory function.56
Pressure-Bounded Coronary Flow Reserve
Pressure-bounded coronary flow reserve (CFRpb) is an index that estimates CFR solely from pressure data acquired during resting and hyperaemic conditions.57 Using fluid mechanics theory, the relationship between flow across a stenosis and the subsequent pressure drop can be explained as:
ΔP = f · Q + s · Q2
where ΔP is the pressure loss, Q is the coronary flow across the stenosis, f is the coefficient of pressure loss due to friction and s is the coefficient of pressure loss due to flow separation.57,58 Using this concept, CFRpb considers the possibility of the pressure gradient loss being either entirely by friction losses or entirely by separation losses, therefore resulting in a value ‘bounded’ by these factors. In practice the equation for CFRpb is:
In the application of CFRpb, both limits should be above or below 2 in order to definitively classify as normal (CFRpb >2) or abnormal (CFRpb <2).57,58 In cases of discordance, CFRpb is deemed to be indeterminate, and therefore pressure cannot be used for the estimation of CFR.58 In one retrospective analysis, CFRpb exhibited 84.4% accuracy to predict flow-derived CFR <2 or ≥2 in 107 lesions.57 However, in a further study with 453 intermediate coronary lesions with combined pressure and Doppler measurements, CFRpb showed poor diagnostic agreement with CFRDoppl.58 Moreover, CFRpb was indeterminate in 43.3% of lesions, and underestimated risk of adverse outcomes over time relative to CFRDoppl.58
Hyperemic Microvascular Resistance
Hyperemic microvascular resistance (HMR) is an index of microcirculatory function also derived using coronary Doppler and pressure wires. It is defined as the ratio of Pd to APV:59
HMR = Pd/APV
The equation is based on the principle of Ohm’s law, where APV is used as a surrogate for flow.
In a study by de Waard et al., an HMR cut-off point of ≥3.0 mmHg/cm/s predicted the occurrence of a composite endpoint of death and hospitalisation following STEMI (HR 7.0), and showed good diagnostic accuracy at predicting cardiac MRI-defined microvascular obstruction (receiver operating characteristic area under the curve (AUC)=0.76).13 Another study of 610 patients, showed that HMR was a predictor of MACE (OR 1.63).60
Minimal Microvascular Resistance
The minimal microvascular resistance (mMR) is a ratio of hyperemic APV to hyperemic Pd, but both measured during the ‘wave-free period’ of diastole, and is independent of epicardial stenosis severity.61,62 Further study of this index is lacking and there are no outcome data in the literature.
Index of Microcirculatory Resistance
In 2006, Fearon et al. published the first work on the index of microcirculatory circulation (IMR), which is a microcirculation-specific index derived using a combined pressure/thermistor coronary guidewire.63 IMR is also derived using the principle of Ohm’s law; however, because IMR is intended to be a microcirculation-specific index, the original equation for its derivation took into account venous pressure:

where Pv is venous pressure and 1/Tm is the inverse of the mean transit time of saline through the coronary.63 Because Pv is low in comparison to Pd, it was subsequently omitted from the equation, as follows:64
IMR = Pd × Tm
In early experimental validation in live open-chest porcine models, IMR was significantly correlated with absolute flow-derived true microcirculatory resistance and appeared to be independent of epicardial stenosis severity.63 Further study revealed that minimal measurable microcirculatory resistance does increase with increasing stenosis severity, but this is not significant when coronary collateral flow is taken into account.65,66 Yong et al. subsequently developed a calculated IMR (IMRcalc) that is accurate in the presence of epicardial stenoses using the equation:66
IMRcalc = Pa × Tmn × ([1.35 × Pd/Pa] – 0.32)
The normal cut-off point for IMR is usually taken as <25, and this is the value also used in the CorMicA study.15,11 However, in STEMI, a value >40 is used to denote severe microvascular dysfunction, and this has been shown to be associated with adverse outcomes, namely rehospitalisation with heart failure, death, microvascular obstruction and the 30-day incidence of pulmonary oedema, malignant ventricular arrhythmias and intraventricular thrombus.67,15,16,68 The reproducibility of bolus thermodilution-derived IMR was subsequently tested in humans and found to be more reproducible than CFRbolus, primarily because it is not influenced by haemodynamic status or fluctuation.55 Despite these qualities, bolus thermodilution-derived indices have been shown have more inter- and intraobserver variability than Doppler-derived indices.53 Furthermore, because IMR is only a hyperaemic index, it does not provide information about microvascular resistance in the resting state.
Resistance Reserve Ratio
The resistance reserve ratio (RRR) is a bolus thermodilution-derived index of microcirculatory function that is defined as a ratio of estimated resting (baseline) microcirculatory resistance to hyperaemic (minimal) microcirculatory resistance.69 RRR reflects the microcirculation’s ability to change from baseline to minimal in response to pharmacologically mediated vasodilatation. As with IMR, the equation for RRR can be modified using the calculation of Yong et al. to account for the presence of an epicardial stenosis. The equation for the derivation of RRR is as follows:

In a study of 1245 patients with stable coronary disease and intermediate stenoses, a reduced RRR <3.5 was associated with an increased risk of patient-oriented composite outcomes, a composite of all-cause mortality, MI and revascularisation at 5 years.70 This was despite a physiologically normal FFR (>0.80) and CFR (>2.0), suggesting that RRR could be included in the risk assessment of coronary artery lesions.
Zero-Flow Pressure
In 2015, Patel et al. described a method of determining the zero-flow pressure (Pzf), defined as the theoretical distal coronary pressure at which flow in the coronary would cease.71 Because this is not practically feasible, the Pzf was extrapolated using pressure–velocity loops acquired using combined invasive Doppler and pressure traces. Pzf was compared to IMR and HMR as a predictor of cMRI-defined infarction size at 6 months following primary PCI. Pzf was found to be superior to HMR and IMR for predicting infarction size (AUCs of 0.94, 0.74 and 0.54, respectively), with an optimal value of ≥42 mmHg.71 Pzf was also a strong predictor of salvage index, 6-month ejection fraction, troponin, final infarct mass, percentage of left ventricular infarction and percentage transmurality of infarction.71 To our knowledge, there are no commercial solutions for the rapid assessment of Pzf in the CCL, and assessment currently relies on post hoc analysis of pressure and flow traces.
Microvascular Resistance Reserve
In 2021, Bruyne et al. first published work on a novel index of microvascular function termed the microvascular resistance reserve (MRR).35 This index had been developed to be specific to the microcirculation and independent of myocardial mass and autoregulation. In essence, the MRR is a ratio of hyperaemic microvascular resistance to true resting resistance, corrected for driving pressures.
True resting resistance is defined as the resistance of the myocardial territory at rest in the absence of epicardial disease, because the microvascular resistance will decrease in response to epicardial disease in order to augment flow.35 The equation for MRR is:35
MRR = (CFR/FFR) × (Parest/Pahyper)
where Parest and Pahyper are resting and hyperaemic aortic pressure, respectively. Validation in humans was conducted in 40 coronary arteries using both a pressure/thermistor wire and a Doppler wire.35 Coronary flow was derived using continuous thermodilution at a low flow (10 ml/min) of saline infusion to derive resting flow and at high flow (20 ml/min) to derive a hyperaemic absolute flow. CFRDoppl was derived using a Doppler wire. MRR derived from Doppler (MRRDoppl) and MRR derived from continuous thermodilution (MRRthermo) showed excellent correlation (r=0.88) and good agreement. However, when CFRDoppl was compared to MRRthermo, the correlation was not as strong (r=0.68). These differences can be explained by the presence of epicardial disease, which CFRDoppl does not correct for.35
This novel index provides an exciting new method for specifically quantifying microcirculatory function; however, further study is required. The upcoming international European microCirculatory Resistance and Absolute Flow Team (EuroCRAFT; NCT04598308) registry will investigate the prognostic utility of MRR on symptoms and hard clinical outcomes.
Angiography-Derived Index of Microcirculatory Resistance
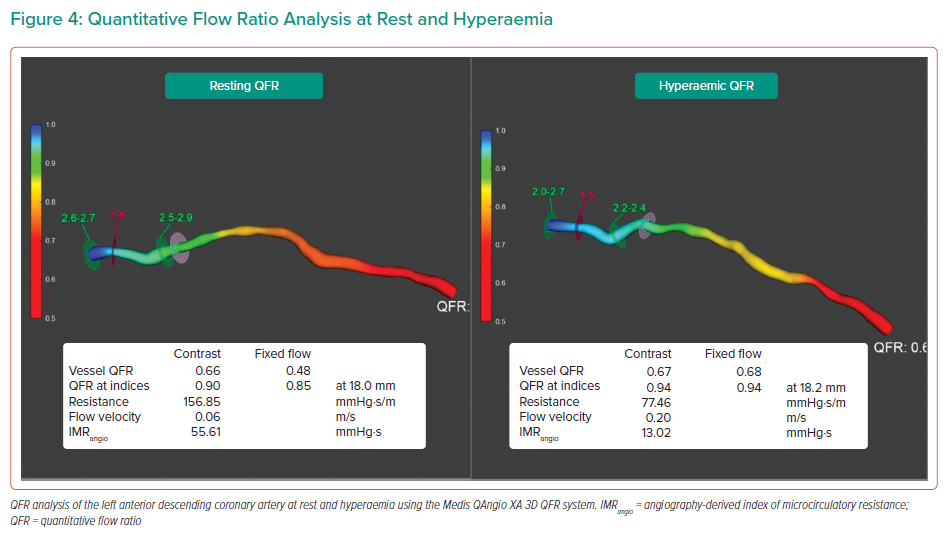
In 2020, De Maria et al. first described a pressure-wire-free method of deriving IMR using angiography-based software.41 In a cohort of 45 patients presenting with STEMI, IMR was measured using the bolus thermodilution technique in conjunction with a pressure/thermistor wire immediately before and after stenting. IMR was also measured in a small subgroup of non-infarct arteries (non-IRA). Immediately following each IMR measurement, while still under hyperaemic conditions, two angiographic images of the coronary artery in question were taken with at least 25° of separation at 15 frames per second (fps) in order to calculate a quantitative flow ratio (QFR) using Medis QAngio 3D QFR software (Figure 4). In a post hoc manner, an angiography-derived IMR (IMRangio) was calculated using the standard IMR equation, IMR = Pd × Tmn, where Pd can be replaced by QFR × Pd, and Tmnwas derived using the transit time of contrast during angiography, calculated by frame counting. The simplified equation for IMRangio is therefore:

IMRangio and IMR were significantly correlated in the total sample of 92 lesions (including pre- and post-stenting and non-IRA; rho=0.85).41 Receiver operating characteristic curves showed excellent diagnostic performance of IMRangio >40 to detect IMR >40 (AUC 0.96).41
Scarsini et al. assessed this method further by expanding its use to other coronary syndrome states including non-STEMI (NSTEMI) and CCS.42 In that study, IMRangio was significantly correlated with invasive IMR in STEMI, NSTEMI and CCS (rho = 0.85, rho = 0.72 and rho = 0.70, respectively).42 IMRangio also showed excellent diagnostic accuracy in predicting IMR >40 in STEMI (AUC = 0.93), and good diagnostic accuracy in predicting an IMR >25 in NSTEMI and CCS (AUC 0.78 and 0.88, respectively).
A recently published systematic review and meta-analysis investigated the diagnostic performance and prognostic impact of angiography-derived IMR.72 That analysis included 1,654 lesions from eight studies, 733 of which were STEMI cases. IMRangio showed a high diagnostic accuracy of predicting invasive IMR, with a pooled sensitivity and specificity of 81% and 80%, respectively. The prognostic impact of IMRangio was investigated in four of the studies, the analysis of which demonstrated that high IMRangio is significantly related to the risk of MACE (HR 2.97).72
Overall, IMRangio represents an exciting pressure-wire-free tool in the investigation of CMD and potentially opens the door of CMD investigation to non-interventional CCLs.
Discussion
The assessment of CMD is an area of growing interest and clinical utility within the field of cardiology, and the need to develop tailored therapies for different population groups is being recognised. This paper has detailed the historical work behind the synthesis of microvascular indices, as well as novel indices and areas of interest.
In describing the methodology underpinning the diagnosis of CMD, we have comprehensively highlighted the advantages and disadvantages of each of the different methods. Doppler, although supported by years of clinical research, is limited by the difficulty in achieving adequate signals. Bolus thermodilution is limited by inter- and intraobserver variability, and has also been shown to overestimate CFR relative to Doppler.50 Pzf calculation currently requires a post hoc analysis of pressure and Doppler traces. Finally, pressure-bounded CFR is limited by its diagnostic accuracy.
In contrast, areas of growth lie within the novel method of continuous thermodilution, due to its ease of use and reproducibility. Moreover, the novel index MRR promises to be a microvascular-specific index of microcirculatory function, but more study is needed to further validate its use. Another area of significant growth lies within the field of computational coronary physiological assessment, and the novel IMRangio represents the first step towards a guidewire-free method of assessing the microcirculation that will be more accessible to the non-interventional cardiologist.
Although research into the diagnosis of CMD has been ongoing for decades, it is worth noting that it has not yielded many significant changes in clinical practice. The CorMicA trial has highlighted the importance of a guided approach to both the investigation and management of microvascular dysfunction, and has further emphasised the need for additional randomised controlled trials in the field.11
In STEMI, no-reflow or poor flow after revascularisation remains a major conundrum for cardiologists to solve, and microvascular injury is postulated to be a central factor in these phenomena. The rapid diagnosis of microvascular injury at the time of STEMI revascularisation is fundamental to the development of targeted therapies such as pressure-controlled intermittent coronary sinus occlusion.73
In the future, it is likely that the invasive assessment of coronary disease will comprise of both epicardial and microvascular assessments in order to arrive at a comprehensive and accurate diagnosis, as well as to tailor medical therapy to individual patients. Beyond the scope of this review, there is also renewed interest in the diagnosis and management of vasomotor and vasospastic coronary disease, which is likely to become an integral part of the diagnostic process in INOCA. Having an up-to-date grasp of the methods of diagnosing CMD will aid in diagnostic confidence and, in turn, in the development of future treatments and understanding of this heterogeneous group.