Renewed interest in renal denervation (RDN) has been justifiably spurred by multiple recent sham-controlled trials demonstrating the sustained lowering of uncontrolled hypertension both in the presence and absence of hypertension medication.1–5 Together with extensive registry-based evidence, this growing body of data indicates meaningful reductions across a broad hypertension population, including subgroups at high cardiovascular (CV) risk.6
The purpose of this review is to summarise the existing recommendations on RDN and to offer practical guidance for adopting RDN in clinical practice. Our interpretation of how RDN should be used is based on both personal experiences and the expanding body of clinical data and guidelines issued by professional societies and working groups.
Review of Current Guidance
Clinical Practice Guidelines on Hypertension
2018 European Society of Hypertension/European Society of Cardiology Guidelines for the Management of Arterial Hypertension
In 2018, the European Society of Cardiology/European Society of Hypertension (ESC/ESH) issued guidelines on the management of hypertension that stated that “the clinical evidence in support of RDN as an effective [blood pressure (BP)] lowering technique is conflicting” and that the “use of device-based therapies is not recommended for the routine treatment of hypertension, unless in the context of clinical studies and [randomised control trials (RCTs)] until further evidence regarding their safety and efficacy becomes available”.7 The ESC/ESH recognised the evidence that supported the safety of RDN and its reduction in sympathetic activity. It also referred to the SYMPLICITY HTN-1 and HTN-2 trials, which demonstrated the BP-lowering efficacy of RDN.8,9 However, the deficit of evidence showing RDN superiority in reducing BP (unless used in combination with optimised pharmacotherapy) led to the ESC/ESH in 2018 holding back from recommending RDN for routine treatment of hypertension until further evidence became available.
2020 International Society of Hypertension Global Hypertension Practice Guidelines
In 2020, the International Society of Hypertension published its global hypertension practice guidelines, which made no mention of RDN.10 Disparities in resources in high- and low-income regions may explain the absence of RDN.
2021 European Society of Hypertension Position Paper on Renal Denervation
Within the 3 years of the 2018 ESC/ESH arterial hypertension guidelines, a set of independent sham-controlled RCTs were completed demonstrating a significant impact of RDN on ambulatory and office BP.11 These new data demonstrated the BP-lowering efficacy of RDN in patients both with and without concomitant hypertension medication. In 2021, the ESC/ESH published an updated position paper on RDN that recognised its role in BP control. The ESC/ESH proposed the following:
- On the basis of the results of sham-controlled clinical trials, RDN represents an evidence-based option to treat hypertension, in addition to lifestyle changes and medication.
- RDN expands therapeutic options to address the first objective of hypertension treatment to effectively reduce an elevated BP and achieve BP targets.
- RDN is considered a safe endovascular procedure without significant short- or long-term adverse effects based on data available up to 3 years.
- A structured pathway for the clinical use of RDN in daily practice is recommended.
- Patients’ perspective and preference, as well as patients’ stage of hypertensive disease, including comorbidities, should lead to an individualised treatment strategy.
2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension
The Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the Management of Hypertension make the following recommendations with respect to RDN in their 2022 guidelines:
…renal denervation should be considered as a BP-lowering strategy in hypertensive patients with high CV risk, such as resistant or masked uncontrolled hypertension, established [atherosclerotic cardiovascular disease], intolerant or nonadherent to antihypertensive drugs, or features indicative of neurogenic hypertension after careful clinical and imaging evaluation.12
This is based upon a review of data from the latest clinical trials, including the SPYRAL HTN-OFF MED and SPYRAL HTN-ON MED trials, RADIANCE-Hypertension (HTN) SOLO and RADIANCE-HTN TRIO trials, and the Global SYMPLICITY Registry, which demonstrate the safety and efficacy of RDN in lowering BP.1–5
The recommendation was graded class IIa, which means the weight of evidence/opinion is in favour of usefulness/efficacy and the recommendation should be considered. The TSOC/THS graded the recommendation as level of evidence B.12
Position and Consensus Statements
Renal Denervation in Patients With Hypertension: Proceedings from an Expert Consensus Roundtable
In 2021, the views of a consensus roundtable, supported by the National Kidney Foundation (NKF) and the Society for CV Angiography and Interventions (SCAI), on the role of RDN as a therapeutic option in addition to medical therapy and lifestyle interventions were published.13 The expert roundtable arrived at the following consensus for RDN safety and efficacy:
- The efficacy of RDN for the treatment of uncontrolled HTN has been consistently demonstrated in sham-controlled randomised trials both in the presence and absence of medications.
- Current evidence with RDN suggests a constant reduction in BP over day and night (‘always on’ effect) that is distinct from pharmacokinetic profiles and dosing regimens with medications and patient non-adherence.
- Both randomised trials and registries support the early- and late-term safety of RDN.
- Although registry data suggest long-term durability in BP reduction following RDN, longer-term surveillance of existing trials and additional studies may inform durability and impact on clinical outcome.
- Clinically useful, reliable predictors of RDN responsiveness need to be identified.
The consensus also proposed criteria for patient populations who may be appropriate for RDN:
- patients with persistent uncontrolled HTN despite the prescription of guideline-based therapy and patients who are intolerant of or unable to remain adherent to their medication regimes;
- patients in whom HTN is confirmed by alternative means of BP monitoring other than office BP measurement alone; and
- patients in whom secondary causes of HTN have been excluded.
Treatment priority is placed on those patients with an elevated CV risk, possibly with established CV event or organ damage.
2022 Malaysian Working Group Consensus Statement on Renal Denervation for Management of Arterial Hypertension
On 1 June 2022, the Malaysian Working Group published its consensus on the use of RDN, taking into account the current data available on RDN as an adjunctive treatment in poorly controlled or resistant arterial HTN.14 Similar to the NKF/SCAI consensus, the Malaysian Working Group consensus recognised the benefits of the ‘always on’ effect of RDN in treating uncontrolled HTN and reducing the impact of medication burden. The Malaysian Working Group made the following recommendations:
- Successful denervation can be an effective adjunctive treatment for sustained lowering of BP.
- Renal denervation should be considered early in the management of HTN.14
The consensus offered guidance to clinicians on selecting patients who will benefit most from RDN as those:
- for whom BP remains high or above target despite full adherence with the maximum appropriate combination of pharmacological agents that can be tolerated;
- with resistant HTN;
- with a history of repeated non-adherence despite numerous counselling sessions;
- on polypharmacy for multiple comorbidities;
- with multiple end-organ damage, with high CV risk;
- unwilling to take long-term pharmacotherapy; and
- with an intolerance to antihypertensive medications.14
2022 Clinical Consensus Statement of the European Society of Cardiology/European Association of Percutaneous Cardiovascular Interventions
In September 2022, the ESC Council on Hypertension announced the need to bring RDN back to the attention of the cardiology community in light of new evidence and prepared a joint clinical consensus statement with the European Association of Percutaneous CV Interventions (EAPCI).15 Since 2018, the ESC has identified several high-quality randomised sham-controlled trials demonstrating BP-lowering efficacy for both radiofrequency and ultrasound RDN in a broad range of patients, along with a meta-analysis of more than 5,000 patients. They make the following broad recommendations:1-6
- RDN may be used in adult patients with uncontrolled resistant HTN, defined as office BP ≥140 mmHg systolic (SBP) or ≥90 mmHg diastolic (DBP), confirmed by 24-h ambulatory SBP ≥130 mmHg or daytime SBP ≥135 mmHg, treated with three or more antihypertensive drugs (including a diuretic) and with an estimated glomerular filtration rate (eGFR) ≥40 ml/min/1.73 m2.
- RDN may be a possible treatment option in patients who are unable to tolerate antihypertensive drugs in the long term and who express a preference to undergo RDN in a shared decision-making process.
The ESC/EAPCI advise that, when considering RDN treatment:15
- the patient’s global CV risk should be evaluated (SCORE and SCORE2-OP in older persons), taking HTN-mediated organ damage and CV complications into account;
- in the absence of evidence, it is not advised to perform RDN (outside of studies) in:
– kidney transplant recipients;
– patients with severely impaired kidney function (KDIGO stage G4 and G5);
– patients requiring haemodialysis;
– patients with fibromuscular dysplasia;
– patients with untreated secondary HTN; and
– patients with a single functioning kidney.
The ESC/EAPCI consensus recommends that the decision-making process should incorporate the preference of a well-informed and educated patient and that there should be optimised shared decision-making with patients and multidisciplinary HTN team care.15
Ultrasound is a highly operator-dependent imaging modality that requires an experienced clinician who is accurately trained with specific skills. Accordingly, the ESC/EAPCI provide the following recommendations for preprocedural imaging:15
- If invasive renal artery imaging is not an option, CT or magnetic resonance angiography (MRA) are preferential to duplex ultrasound.
- Selective renal angiography immediately before RDN remains the gold standard because CT angiography or MRA may miss some renal artery abnormalities.
Review of Clinical Evidence
Although several initial studies demonstrated significant BP reduction following RDN, this came under doubt with the results of the SYMPLICITY HTN-3 trial in 2014.16 SYMPLICITY HTN-3 was the first randomised sham-controlled trial to be performed. However, it did not show significantly lower office or 24-h ambulatory SBP compared with sham treatment with a pre-specified superiority margin.16
Scrutiny of SYMPLICITY HTN-3 reveals several factors that may explain why the intervention failed to demonstrate benefit. The reasons are multifactorial, but the biggest confounder is understood to be variable medication adherence throughout the course of the study.1,17–19 Additional problems include the lack of experience of operators with the SYMPLICITY device, procedure variability and the inability of the first-generation device to allow four ablations to be performed simultaneously.1,16
Second-generation studies have adopted a number of measures to overcome these limitations:
- primary endpoints that measure the change in ambulatory rather than office BP;
- rigorous screening procedures to identify appropriate patients for RDN;
- the use of newer multisite denervation systems;
- better procedural techniques; and
- objective adherence testing.16
SPYRAL HTN-OFF MED (SPYRAL Pivotal)
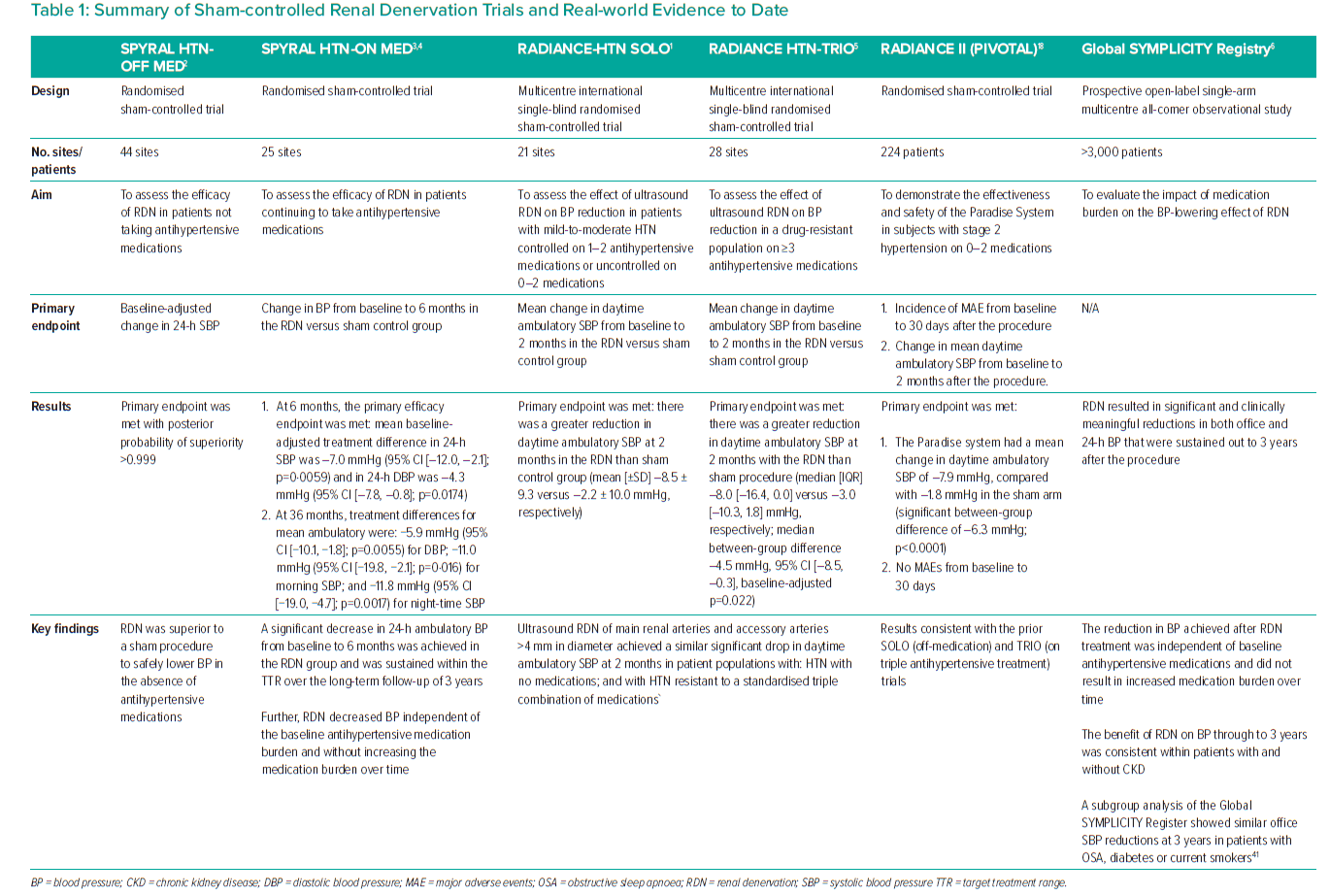
The SPYRAL HTN-OFF MED study was a randomised sham-controlled trial conducted across 44 sites designed to assess the efficacy of RDN in patients not taking antihypertensive medications.2 It performed RDN with the second-generation radiofrequency ablation system using an ablation protocol that included treatment of the distal renal artery as well as the branch renal arteries.2,16 The primary efficacy endpoint was baseline-adjusted change in 24-h SBP, and the secondary efficacy endpoint was baseline-adjusted change in office SBP from baseline to 3 months after the procedure.2 Both primary and secondary efficacy endpoints were met with posterior probability of superiority >0.999 for both (Table 1).
The results of the SPYRAL HTN-OFF MED study demonstrate the superiority of catheter-based RDN compared with a sham procedure to safely lower BP in the absence of antihypertensive medications.
SPYRAL HTN-ON MED
The SPYRAL HTN-ON MED pilot trial was a randomised sham-controlled trial conducted across 25 sites in patients continuing to take antihypertensive medications.3 RDN was performed using the second-generation radiofrequency ablation system with an ablation protocol that included treatment of the distal renal artery and the smaller branch renal arteries, although not into the renal parenchyma. Eligible patients had an office SBP of between 150 and 180 mmHg and a DBP of ≥90 mmHg; a 24-h ambulatory SBP of between 140 and 170 mmHg at the second screening; and were taking between one and three antihypertensive medications with stable doses for at least 6 weeks (Table 1).
The primary efficacy endpoint was the change in BP from baseline (measured at the second screening visit), based on ambulatory BP measurements assessed at 6 months, compared between the RDN and sham control groups.3 The primary efficacy endpoint was met, as demonstrated by a significant decrease in 24-h ambulatory BP from baseline to 6 months in the RDN group (mean baseline-adjusted treatment differences: 24-h SBP, −7.0 mmHg, 95% CI [−12.0, −2.1], p=0.0059; 24-h DBP, −4.3 mmHg, 95% CI [−7.8, −0.8], p=0.0174; Table 1).
Efficacy analysis of this population, along with medication usage and safety, was continued and assessed up to 36 months.4 Treatment differences between the RDN and sham control groups at 36 months were −11.0 mmHg (95% CI [−19.8, −2.1]; p=0.016) for morning SBP, −11.8 mm Hg (95% CI [−19.0, −4.7]; p=0.0017) for night-time SBP and −5.9 mmHg (95% CI [−10.1, −1.8]; p=0.0055) for mean ambulatory DBP.4 The biggest sham-adjusted drop in SBP occurred at night (Supplementary Material Figure 1).4
Most recently, 6-month results from the expansion cohort were released.20 Although the primary endpoint of 24-h ambulatory BP did not differ significantly between the device and sham groups, this was attributed to an unexpected drop in BP in the sham group, mediated through changes in patient behaviour during the COVID-19 pandemic, which affected more than 80% of patients in the expansion cohort. Specifically, significant differences in baseline 24-h ambulatory BP patterns between the pre- and during-COVID-19 populations were observed, and patients in the sham group increased their medication during this period. Despite this, the RDN group met its primary safety endpoint, with a low incidence of procedure-related and clinical adverse events at 6 months, as well as prespecified secondary endpoints of a reduction in office SBP (treatment difference of −4.7 mmHg; p=0.001); and the win ratio, which combined BP reduction with the reduction in medication burden (p=0.005). The overall burden of medications was significantly higher in the sham group at 6 months (p=0.04).20
Overall, SPYRAL HTN-ON MED showed a clinically meaningful reduction in BP with significantly higher time in the treatment range (up to 36 months) in the RDN compared with the sham group, independent of concomitant antihypertensive medications, thus affirming the sustained efficacy of RDN over long-term follow-up.4
RADIANCE−HTN
In the RADIANCE-HTN trial series, ultrasound RDN was used in two patient populations: a population with uncontrolled HTN after a 4-week discontinuation of up to two antihypertensive medications (RADIANCE-HTN SOLO); and a drug-resistant population on three plus antihypertensive medications (RADIANCE-HTN TRIO).15 The data from the two cohorts showed that there was no heterogeneity in the effect on BP reduction and medication burden. The data from a mixed population over a 6-month period demonstrated that ultrasound RDN had greater efficacy over the sham population and the pooled analysis of the RADIANCE-HTN trial cohorts supports “the response to ultrasound RDN is similar in the presence or absence of medications and consistent across the whole spectrum of hypertension”.15
RADIANCE-HTN SOLO
RADIANCE-HTN SOLO was a multicentre international single-blind randomised sham-controlled trial performed at 21 centres on patients with HTN in the absence of antihypertensive medications (Table 1).1 The primary efficacy endpoint of RADIANCE-HTN SOLO was the mean change in daytime ambulatory BP at 2 months between patients with mild to moderate HTN off antihypertensive medications who underwent endovascular ultrasound RDN and those who underwent a sham procedure. In this trial, the primary endpoint was met. In the intention-to-treat population, there was a greater reduction in daytime ambulatory SBP at 2 months in the renal denervation group than in the sham group (mean [±SD] −8.5 ± 9.3 versus −2.2 ± 10.0 mmHg, respectively; Table 1; Supplementary Material Figure 2).
RADIANCE HTN-TRIO
RADIANCE HTN-TRIO was a randomised international multicentre single-blind sham-controlled trial performed in 28 tertiary centres in the US and 25 in Europe (Table 1).5 Participants had resistant HTN, defined as seated office BP of at least 140 mmHg systolic and 90 mmHg diastolic despite a stable regimen of three or more antihypertensive medications including a diuretic, and an eGFR ≥40 ml/min per 1.73 m². As in RADIANCE HTN-SOLO, the primary efficacy endpoint was the change in daytime ambulatory SBP from baseline to 2 months. In the intention-to-treat population, there was a greater reduction in daytime ambulatory SBP at 2 months with RDN compared with the sham procedure (median [IQR] −8.0 [−16.4, 0.0] versus −3.0 [−10.3, 1.8] mmHg; median between-group difference −4.5 mmHg, 95% CI [−8.5, −0.3 mmHg]; baseline-adjusted p=0.022; Table 1; Supplementary Material Figure 3).5
The RADIANCE-HTN TRIO trial was designed to overcome the limitations of previous studies in resistant HTN by adjusting participants’ antihypertensive treatment in line with current guidelines and reducing the pill burden to achieve a high adherence at baseline that was maintained at 2 months in both groups.5
RADIANCE II (Pivotal)
RADIANCE II Pivotal was a randomised sham-controlled clinical trial that assessed the effectiveness and safety of the Paradise System in 224 patients with uncontrolled mild-to-moderate HTN and not on antihypertensive medication during the trial.17 Eligible patients had no type 1 or uncontrolled type 2 diabetes, no prior CV or cerebrovascular events and, after a 4-week medication washout period, were required to have daytime BP of at least 135/85 mmHg but <170/105 mmHg. After screening, patients were randomised 2:1 to RDN or sham control and followed for 2 months (Table 1).
At the 2-month primary efficacy endpoint, patients treated with the Paradise system had a mean change in daytime ambulatory SBP of −7.9 mmHg, compared with a change of −1.8 mmHg in the sham arm, corresponding to a statistically significant between-group difference of −6.3 mmHg (p<0.0001).17 Reductions were observed in night-time and 24-h measures, as well as measurements taken at home and in the physician’s office. No major adverse events were seen at 30 days; the primary safety endpoint will be measured at 6 months, and patients will be followed for 60 months.
Global SYMPLICITY Registry
The Global SYMPLICITY Registry is the largest outcome research project, with the longest follow-up (3 years), that collected and analysed (and continues to collect and analyse) real-world data on the safety and efficacy of RDN in patients with uncontrolled HTN or another condition associated with increased sympathetic activity (Table 1).6 At baseline, office SBP was 166 mmHg and 24-h SBP was 155 mmHg; patients were taking, on average, 4.6 different classes of antihypertensive medication.6 To date, the registry has enrolled over 3,100 patients treated with RDN using either the original Symplicity Flex™ catheter or the newer-generation Symplicity Spyral™ catheter. More than 2,500 patients were included in the 3-year follow-up that evaluated the impact of medication burden on the BP-lowering effect of RDN.4
Significant and clinically meaningful reductions in office BP and ambulatory BP monitoring were achieved with RDN that were sustained to at least 36 months for patients who had RDN treatment. The reduction in BP achieved following RDN treatment was independent of baseline antihypertensive medications and did not result in an increased medication burden over time. The BP reduction was similar and consistently observed in patients with severe resistant HTN (defined as baseline office SBP >160 mmHg despite the prescription of three or more antihypertensive drug classes), type 2 diabetes, elderly subjects, patients with chronic kidney disease and those with isolated HTN.6
Long-Term Follow-up of Patients Undergoing Renal Denervation
Long-term follow-up of patients who have received RDN procedures include a late follow-up of the original SYMPLICITY patients (out to 5 years), the SYMPLICITY Registry (out to 3 years), the SPYRAL HTN-OFF and HTN-ON MED studies (out to 36 months) and the RADIANCE trials (out to 36 months).1-6 In these studies, there appears to be a trend to lower BP over time. This could be due to the addition of antihypertensive medications over time, but, remotely, there may be a gradual late effect of the RDN procedure. Ongoing data collection on these trials will provide further clarity to the question of the long-term efficacy and safety of RDN but, to date, existing data leave little doubt as to the value of RDN in uncontrolled HTN. The principles of identifying and selecting appropriate patients are discussed below.
Practical Guidance
Which Patients Should Be Considered for Renal Denervation?
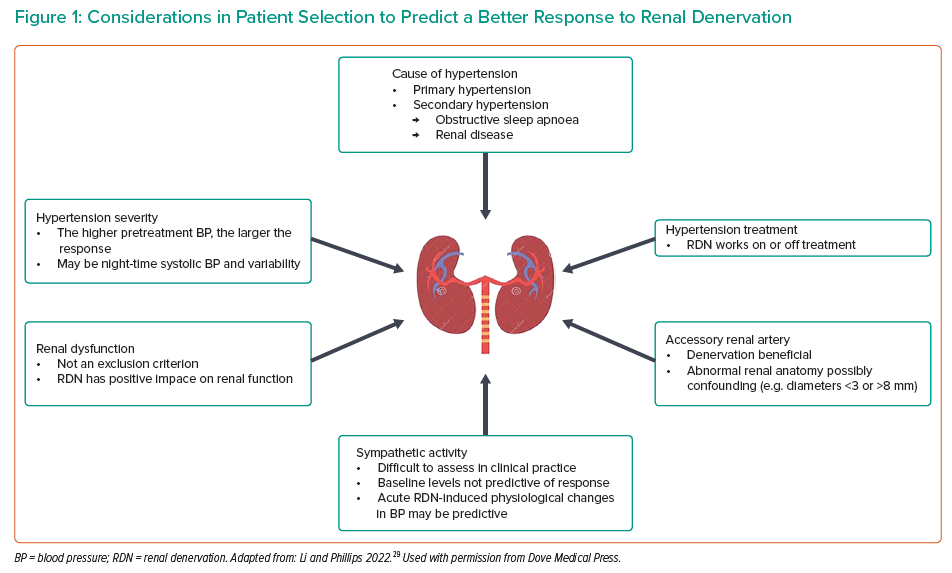
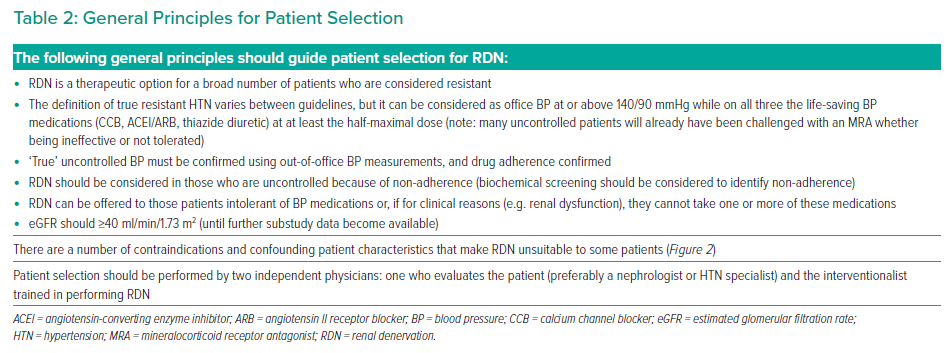
There is currently no validated predictor of BP response to RDN, although several physiological measures show some correlation. These can be divided into two categories: parameters associated with lower arterial stiffness and parameters associated with increased basal sympathetic nervous activity. The former include aortic pulse wave velocity, augmentation index and aortic distensibility.21–25 The latter include plasma renin activity, elevated ambulatory heart rate and variability of baseline night-time SBP.21–25 In the absence of strong predictive features and based on increasing evidence, RDN can be considered in a wide range of patients (Figure 1). Suitable patient groups reflect guidance issued from recent position statements, which have tended to include patients who are at high CV risk as well as those deemed non-adherent to HTN medication.11,15
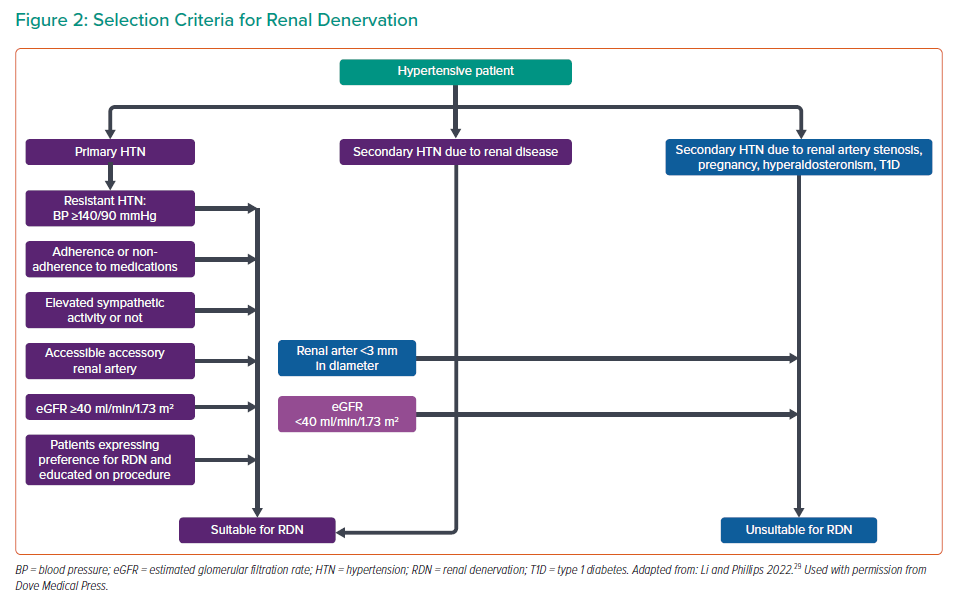
It is important to highlight specific patients in whom RDN may not be suitable at this time. RDN may be best avoided in patients with an eGFR <40 ml/min/1.73 m2 because an eGFR between 45 and 40 ml/min/1.73 m² was the cut-off used in many trials to date.26–28 In addition, patients with HTN secondary to renal artery stenosis and patients with a renal artery diameter <3 mm and the presence of structural renal abnormalities need careful consideration for RDN; these factors could preclude proper ablation of the renal nerves and undermine the therapeutic effects of RDN. Of note, trials performed with the Symplicity Spyral system did not exclude patients with smaller vessel sizes.2,4 RDN is also unsuitable for patients with secondary HTN from causes such as pregnancy, primary aldosteronism or valvular heart disease.29
An algorithm summarising selection criteria for RDN is presented in Figure 2.
Resistant Hypertension
‘True’ resistant HTN is when a patient has an elevated office BP above goal on at least three antihypertensive drugs, confirmed by out-of-office measurements and with (confirmed) good adherence to antihypertensive therapies. ‘True’ resistant HTN should be differentiated from ‘apparent’ resistant HTN (defined as lack of control on three or more medications and where pseudo-resistant HTN cannot be excluded).30
Generally, resistant HTN can be considered as SBP at or above 140 mmHg or DBP above 90 mmHg, measured correctly on three separate occasions and at least 2 weeks apart. Three of the drugs should include an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, a diuretic and a dihydropyridine calcium channel blocker at maximum tolerated doses.
Attention should be paid to the differing and evolving definitions of resistant HTN. First, guidelines differ in their resistant HTN threshold, which may be as low as 130/80 mmHg.12 The most recent ESC/ESH guidelines define resistant HTN as the failure to achieve BP ‘control’ to levels below 140/90 mmHg despite treatment with three antihypertensive agents with complementary mechanisms of action (with at least one diuretic).7 The American Heart Association guidelines adopt an extended definition to include patients with four or more antihypertensive medications, even when BP is controlled (controlled resistant HTN).31 Thus, the term ‘resistant HTN’ may include both uncontrolled and controlled BP, depending on the number of antihypertensive agents used.32 For context, resistant HTN accounts for 5–30% of the overall hypertensive population, controlled resistant accounts for 20% of the total population and refractory HTN accounts for approximately 5% of the resistant population.33
Despite nuanced terminology, all resistant HTN labels identify patients who are at higher risk of CV morbidity and mortality. Indeed, there is a direct correlation between the extent of resistant HTN and the number of events.33 As such, patients with apparent resistant HTN should be identified early because they may benefit from special therapeutic approaches, including RDN.
Uncontrolled Hypertension Due to Non-adherence
Only 25–34% of patients comply with antihypertensive treatment, and up to half of all patients may be at least partially non-adherent.34 Medication adherence is generally lowest among younger patients, those on polypharmacy and in those who develop medication-related adverse events. All these groups are potentially good candidates for RDN, and patient preference should play a role in the decision-making process.
Novel biochemical analyses using liquid chromatography–tandem mass spectrometry can detect up to 40 of the most common antihypertensive medications, and such an approach may be used to assess adherence or non-adherence to the medication regimen.34 Modelling of biochemical screening suggested that adoption prevented 518 MIs and 305 stroke events in a cohort of 10,000 male hypertensive patients.35
Therefore, the persistent BP-lowering effect of RDN may offer additional benefits in partial and even fully non-adherent hypertensive patients. Yet, adherence to antihypertensive drugs remains crucial because most patients still need medications to achieve BP targets.
True medication intolerance is another good reason to consider RDN as a treatment option. This should include intolerance to at least one of the three aforementioned medication classes.
The general principles for patient selection are summarised in Table 2.
How Should Blood Pressure Be Measured?
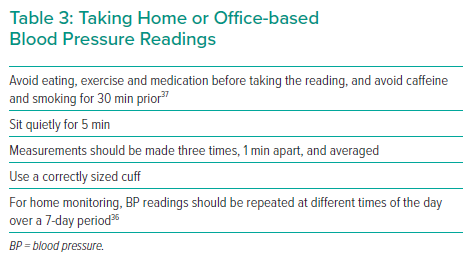
True resistant HTN needs confirmation of elevated BP values outside of the physician’s office. Ambulatory BP monitoring (ABPM) is the preferred method, but this may not be widely available in all countries or, as in the US, not reimbursed by insurers. Therefore, home-based BP monitoring is the best alternative and is preferred over office-based BP monitoring to exclude white coat HTN (Table 3).
However, patients may lose interest in performing BP monitoring over time, so it is important to provide careful instruction, to use recommended simplified protocols and to reinforce proper instruction at regular intervals.36 Home-based monitoring also incurs a financial burden and may not be viable for those of a lower socioeconomic status, although these patients often have the greatest need for BP control.13 Out-of-office BP can also be monitored in other healthcare settings, such as nurse consultations or pharmacists.
How Should Adherence Be Assessed?
There are a number of methods by which medication adherence can be measured, including self-report questionnaires, therapeutic drug monitoring, electronic devices and pick-up/refill rates. It is recommended that multiple methods to measure adherence are combined, keeping individual (dis)advantages for each method in mind.38 Currently, urine analysis is being used as a means to measure medication levels, with samples sent away to reference labs.39
What Are the Current Goals for Blood Pressure Control?
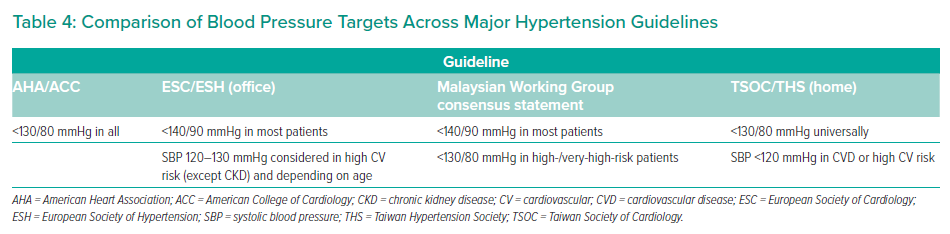
There is some variability between guidelines on BP goals, which may depend on age, the presence of comorbidities and treatment tolerability (Table 4). Despite some differences, the overarching objective of preventing atherosclerotic CV disease and cerebrovascular disease is common among all guidelines.
Does Renal Denervation Improve Long-term Outcomes?
Numerous meta-analyses and prospective trial data support intensive BP lowering for improved long-term CV outcomes: in the most recent meta-analysis of >340,000 individuals from 48 randomised clinical trials, a 5-mmHg decrease in office SBP reduced major CV events by 10%, stroke by 13%, ischaemic heart disease by 8%, heart failure by 13%, CV mortality by 5% and all-cause mortality by 2% after a median 4.2 years follow-up; the risk reduction did not differ across age, sex or baseline SBP categories.40
However, for RDN, due to the absence of CV outcome-based trials, it is not currently possible to answer the question of whether RDN improves long-term CV outcomes, so one must assume that (all being equal) BP lowering through RDN follows the same principle as pharmacological-based lowering with respect to long-term CV benefit. That said, based on the Global Symplicity Registry data, major CV events and stroke occurred in 9.9% and 4.5% of patients with completed follow-up of 3 years.6,11 The absolute risk reduction of major CV events and stroke was estimated at 5.2% in resistant HTN and, on that basis, some consensus statements consider RDN as an option to improve CV outcomes and BP over time.11,15
Reimbursement: What is the Actual Situation?
At the time of writing, RDN is approved on a device-specific basis across Europe and some other countries, excluding the US. In the US, the procedure is approved for investigational use only for both current commercially available devices.
The Symplicity Spyral RDN system (Medtronic) is approved for commercial use in more than 60 countries. It is limited to investigational use in the US, Japan and Canada. The Paradise system (ReCor Medical) is an investigational device in the US. In Europe, the device has received CE Mark approval.
In Europe, the reimbursement situation varies between countries. It is hoped that in the near future, emerging trial results will provide the necessary evidence for US Food and Drug Administration (FDA) approval and advance reimbursement across Europe.
What Information Should Be Imparted to Patients Who Are Considering Renal Denervation?
In line with current position statements, a shared decision-making approach is essential, using patient preference in combination with physician-assessed suitability. Consideration should be given to patient preferences given historical challenges with HTN medication adherence, and the need for better CV prevention strategies.
Market research shows that patient perceptions of RDN as a BP-lowering strategy differ to those of physicians.41,42 In a retrospective analysis of 2,768 patients and 1,902 physicians across Western Europe and the US, patients most likely to accept RDN had a greater understanding of the risks associated with prolonged HTN, had personal experience of the consequences of HTN or had experienced medication side effects themselves.43 Another cross-sectional survey found that a significant proportion of patients would prefer RDN over lifelong medication.43 However, physicians appear most likely to recommend RDN only in the most severe cases and for patients on multiple medications, regardless of patient preference.42
Given the broad number of patients that RDN may be appropriate for, understanding patient perspectives is expected to facilitate the more efficient use of treatments and improve access to suitable treatments.42 However, it is also important for healthcare providers to manage expectations and provide current advice; other patient-based surveys show unrealistic patient expectations with respect to the BP-reduction potential of RDN.43 Studies show that a reduction of only 5 mmHg is enough to prevent CV events, a benefit that patients are willing to accept.40,44 However, patients generally do not receive adequate education around this relationship, so it is incumbent on the physician to provide it.
Outside of more general patient education on the relationship between HTN and CV outcomes, the following messages are essential to communicate to those considering RDN:
- RDN is equivalent to at least one potent medication.
- Patients are unlikely to become medication free.
- Patients should expect to see some reduction in BP by 2–3 months, but the effect improves over time.
- There are potential complications for any invasive procedure.
In addition, what the RDN procedure entails should be explained to patients, including:
- femoral/groin access;
- procedure length 1–1.5 h;
- possible overnight stay for surveillance;
- there may not be an immediate fall in BP; and
- aspirin for 1 month after the procedure.
Additional Management Considerations for Those Undergoing Renal Denervation
After the procedure, immediate care should mirror that for most other catheter-based interventions. For follow-up, we will know more about possible renal artery complications as data are published, but this is unlikely to be a problem unless the patient had atherosclerotic renal artery stenosis at the time of the procedure. As such, follow-up procedures should be as usual for hypertensive patients but incorporate imaging:
- Immediately after RDN:
- aspirin may be indicated, but not long-term;
- patients should be observed for groin access or other complications according to local practice for comparable procedures, and this may involve an overnight stay; and
- patients should be encouraged to maintain their ongoing antihypertensive medication(s) unless they have signs of postural hypotension, in which case they should contact their physician.
- Follow-up:
- should occur at 1, 3, 6 and 12 months after the procedure and should be coordinated between the patient’s general physician and the interventionalist/specialist;
- should monitor office and out-of-office BP to check for BP control, as well as potassium (general physician);
- should include ultrasound imaging at 6 months and 1 year to check for renal stenosis (specialist); and
- should check drug adherence periodically (general physican and specialist).