In the 10 years since the original randomised controlled clinical trials were published examining the role of transcatheter aortic valve replacement (TAVR) in the treatment of severe symptomatic aortic stenosis, TAVR devices have evolved and the choice of TAVR devices has grown at an exponential rate. The TAVR procedure has also evolved, with an emphasis on simplified procedures that involve less frequent balloon valvuloplasty (BAV) of the native valve, less rapid pacing with less frequent need for transvenous temporary pacing wires, and increasing use of the radial approach for non-therapeutic access. BAV, once considered a mandatory step, is now reserved for specific circumstances, as is postdilation of TAVR prostheses. As such, an individualised approach to the TAVR procedure is required. Given the continually growing use of, and knowledge about, TAVR and its extension to low-risk patients, it seems appropriate to revisit the evidence available on BAV and balloon postdilation (BPD). Therefore, the aims of this review are to consider the current data and to identify in which patients and situations their use should be recommended.
Pre-dilation
BAV was initially considered a mandatory step when performing TAVR. The first randomised controlled trials demonstrating the efficacy of TAVR versus conservative management included BAV as part of the implantation technique and treatment of the control group.1 The mechanical effects of BAV (fracturing of the calcium and separation of fused cusps) increase the orifice area to theoretically allow smoother passage of the TAVR prosthesis through the stenosed valve, prepare the valve for TAVR implantation, and ensure uniform expansion of the prosthesis by decreasing radial counterforces, thereby avoiding paravalvular leak (PVL) or valve malposition. In particular, self-expanding TAVR valves often have a lower radial force and may be underexpanded when deployed without predilation, especially in severely calcified aortic valves. Successive iterations of TAVR devices have, however, come some way in resolving these potential issues, and direct TAVR implantation is now more frequently used. Lower profile delivery systems allow smoother passage of the prosthesis across the stenosed valve, and stronger radial force with better expansion of the valve within the annulus as well as improved understanding of aortic valve assessment using 3D echocardiography or CT imaging has led many operators to consider BAV no longer mandatory. Operator experience also plays a role, with a trend towards decreased BAV use in more experienced centres.2
A number of registries and meta-analyses have suggested that pre-BAV could perhaps be omitted. In 2011, Grube et al. published the first series of 60 patients who had been prospectively enrolled and who had undergone direct CoreValve implantation, and compared them with a retrospective cohort of patients who had undergone pre-BAV.3 Procedural technical success, as defined by the first Valve Academic Research Consortium (VARC) criteria, was 96.7%, with BPD being required in 16.7%.4 Thereafter, a number of registries, matched studies and meta-analyses demonstrated similar device success in patients undergoing BAV versus direct TAVR in studies with balloon-expandable prostheses, self-expandable prostheses, and in studies containing both types.2,5–14 The result has been a progressive decline in the use of BAV before TAVR, with many registries demonstrating direct TAVR implantation in approximately 50% of patients.2,15
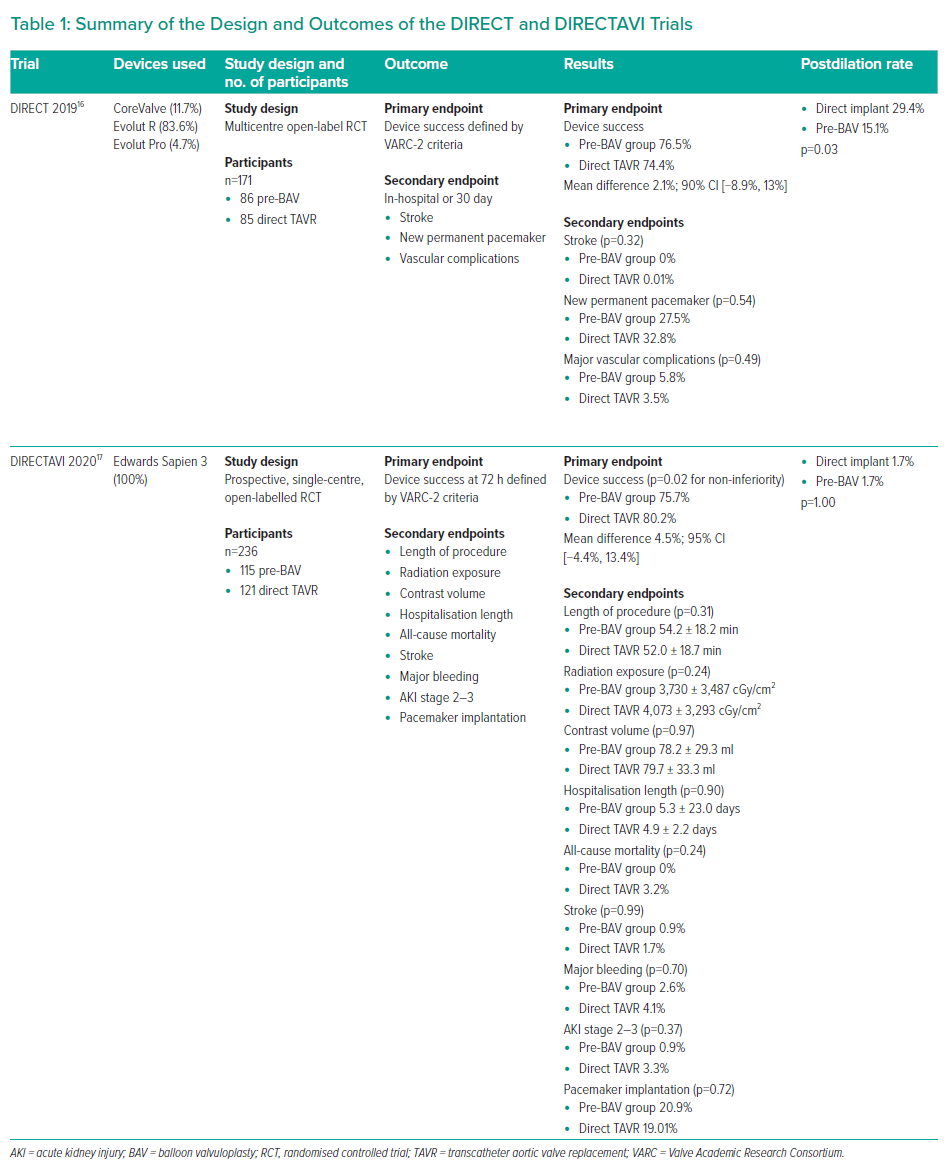
Given, however, that many of the studies included in these meta-analyses were registries without randomisation or prespecified criteria for pre-BAV versus direct TAVR, it is inevitable that there exists a selection bias in terms of those patients who underwent BAV versus those who had direct TAVR. It was therefore not until recently, with the publication of the DIRECT and DIRECTAVI trials, that randomised controlled trial data on this issue became available (Table 1).16,17
The DIRECT trial was a multicentre, open-labelled randomised controlled trial of 171 patients randomised either to pre-BAV (86 patients) or to direct TAVR implant (85 patients) with the self-expanding CoreValve system (mostly Evolut R generation, Medtronic). Device success, as defined by the VARC-2 criteria, was found to be similar in both groups, with direct implantation being non-inferior to implantation after BAV (76.5% in the pre-BAV group versus 74.4% in the direct implant group; mean difference, 2.1%; 90% CI [−8.9%, 13%]).16,18
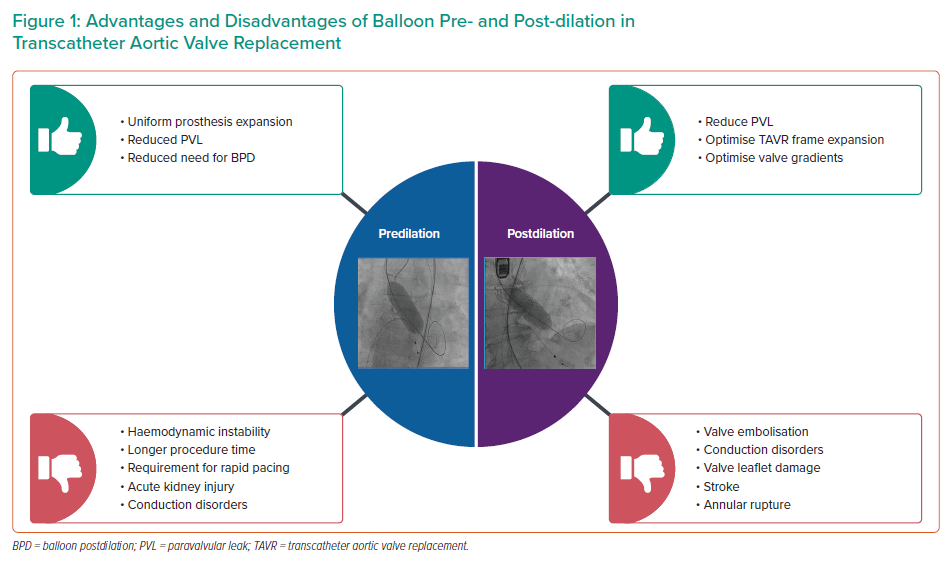
Similarly, the DIRECTAVI trial was a single-centre randomised controlled trial randomising patients to pre-BAV versus direct TAVR with a balloon-expandable prosthesis (Edwards Sapien 3, Edwards Lifesciences).17 Again, device success was similar, and non-inferior for direct implantation versus pre-BAV (80.2% for direct implantation versus 75.7% for pre-BAV; mean difference, 4.5%; 95% CI [−4.4%, 13.4%]; p=0.02 for non-inferiority). It should be noted that crossover to pre-BAV was required in 5.8% of the patients (43% of cases were due to failure to cross the valve, and 57% were based on complex anatomy such as bicuspid valve, low aortic valve area [AVA] and high valve calcium score).17 Other studies have found additional benefits to the omission of pre-BAV including simplification of the TAVR procedure, resulting in reduced procedure time, reduced contrast volume, fewer cases of acute kidney injury (AKI) and reduced fluoroscopy time; these findings, however, were not replicated in the DIRECTAVI trial.5,6,12,17,19,20 As such, pre-BAV is likely to remain an important step for some patients, and understanding the potential risks and benefits is essential when choosing the TAVR strategy for an individual patient (Figure 1). These will be discussed in the following sections.
Potential Risks of Predilation
Haemodynamic Instability
Haemodynamic instability is often cited as an associated risk of BAV. BAV is performed under rapid pacing, which can result in haemodynamic instability, as can the often severe aortic insufficiency that occurs due to separation of the aortic commissures. Prior to the widespread use of TAVR, BAV as a treatment for aortic stenosis resulted in severe aortic regurgitation in 1–2% of patients.21 Furthermore, the EASE-IT TA registry of patients with trans-apical TAVR using the Sapien 3 valve found a lower requirement for catecholamine use in patients who had direct TAVR implantation, suggesting a more balanced haemodynamic state (catecholamine use, 17.5% in the direct TAVR group versus 32.8% in the BAV group, p=0.017).22
However, the same finding was not replicated in the EASE-IT TF registry and, furthermore, in an analysis of the Brazilian TAVR registry, Bernardi et al. reported haemodynamic instability related to valve positioning in those patients undergoing direct TAVR implantation in 2.8% of cases.6,14 Nonetheless, greater troponin rises have been noted with predilation and rapid pacing, suggesting that the haemodynamic effects of these steps can result in myocardial damage, which should be considered, particularly in patients who have baseline ventricular dysfunction.12,23
Conduction Disorders
The close relationship between the aortic valve annulus and the atrioventricular conduction system explains the potential risk of conduction disorders during and after TAVR implantation.24 The His bundle penetrates the membranous septum, the superior border of which lies at, or just superior to, the aortic annulus. The inferior border of the membranous septum marks the transition from the penetrating to the branching segments of the His bundle.24 Mechanical damage and resulting oedema and compression to the atrioventricular conduction system can result in transient or permanent conduction disorders after TAVR. Post-TAVR conduction abnormalities have been associated with poorer outcomes in terms of both rehospitalisation and increased mortality.25
Omission of pre-BAV was thought to reduce the permanent pacemaker (PPM) rate. In fact, in the EASE-IT TF registry the perceived risk of atrioventricular block was cited as the reason for avoiding pre-BAV in 22% of cases planned for implantation of the Edwards Sapien 3 valve.6 Nuis et al. have previously shown that 46% of new conduction disorders occurring during implantation of the CoreValve system occurred after BAV, with a high proportion persisting at discharge; and similarly, an analysis of the Brazilian TAVR registry demonstrated higher rates of new-onset left bundle branch block (LBBB) that persisted at discharge in patients who received a CoreValve after pre-BAV.14,26
A ‘two-hit model’ was proposed by Lange et al., in which the conduction system received a first hit during BAV (resulting in inflammation and intramural haematoma), with the second hit being valve implantation.27 In that study, smaller balloon sizes used for BAV were associated with a lower PPM requirement, a so-called ‘moderate’ predilation approach (PPM rate, 27.1% after predilation with a 25 mm balloon versus 15.4% for a 23 mm balloon, p=0.04), in keeping with the findings by Nuis et al. of higher rates of conduction abnormalities with higher balloon:annulus ratios.26,27 Additionally, Grube et al. in their initial study noted a lower PPM rate in the group with direct TAVR implantation compared with the historic group of patients with BAV (11.7% versus 27.8%, respectively).3 In contrast, some meta-analysis and registry studies have reported either decreased PPM rates or a neutral effect of BAV on pacemaker rates.5,10,11,13 Furthermore, neither DIRECTAVI nor the DIRECT trial had increased PPM rates in their pre-BAV arms (Table 1).16,17 Although not proven in randomised studies to increase PPM risk, it is wise to consider other known contributing factors such as valve choice, and pre-existing conduction disorders before deciding on the final procedural plan and whether this should include pre-BAV or not.28,29
Acute Kidney Injury
Theoretically, hypotension caused by rapid pacing, increased procedural time and increased contrast use in cases of balloon sizing can contribute to kidney injury. A meta-analysis of 18 studies by Liao et al. demonstrated reduced contrast requirement (by ~20 ml) with a tendency to reduced AKI (p=0.08) in patients who had direct TAVR implantation compared with those who had pre-BAV.10 Other studies have also found a reduced requirement for contrast use: the SOURCE 3 registry reported less contrast use in the direct TAVR group by 4.8 ml, which did not translate into differences in AKI (1.4% versus 0.5%, p=0.069 for direct TAVR versus pre-BAV, respectively), while a study by Bijuklic et al. also found that the direct TAVR group had a reduced contrast use by ~17 ml.5,30 However, the randomised DIRECTAVI trial using a balloon-expandable valve did not find a difference in contrast volume used or in AKI between the direct implantation and predilation groups.17 Nor were there differences in AKI between groups in the DIRECT trial.16 In unrandomised trials, predilation with increased contrast use during the procedure may be more reflective of complex anatomy rather than a specific requirement for more contrast with predilation. Furthermore, balloon sizing may be performed with a contrast injection while the balloon is inflated to assess for aortic regurgitation and aid in choosing the prosthesis size. However, this practice is now much less commonly used now that CT imaging is routinely performed. In our institution, balloon sizing is rarely used, and in cases in which predilation is performed, additional contrast injections are not used.
Stroke
Cerebral embolisation of debris during TAVR implantation has been a concern since the first TAVR valves were implanted. The rate of 30-day postprocedure stroke ranges between 2% and 4% in large registries and trials.31–33 Excessive manipulation of the native valve during TAVR is thought to contribute to the periprocedural stroke risk, and this is supported by studies using cerebral protection devices in which cardiac tissue including from valve leaflets, aorta and myocardium has been found on histological assessment of captured debris.34 However, in the aforementioned study by Van Mieghem et al., pre-TAVR BAV was not associated with increased debris in the cerebral protection device.34 Furthermore, other single-centre and registry studies have not shown an association between pre-BAV and stroke at 30 days, and nor have the recent DIRECTAVI and DIRECT randomised controlled trials (predilation versus direct TAVR, 0.9% versus 1.7%, p=0.99 in the DIRECTAVI trial and 0% versus 0.01% in the DIRECT trial).6,17,32 Transcranial Doppler studies identifying cerebral embolisation during TAVR suggest that device positioning and BPD of TAVR valves may be a more important predictor of cerebral embolisation of particulate matter.35,36 Also, BPD has been associated with higher rates of clinically evident cerebrovascular events.33,37 Thus, avoiding predilation to reduce the potential risk of cerebral embolisation during BAV may not be justified if the BPD rate increases in a direct TAVR approach, which has been seen in a number of studies including the DIRECT trial.11,16,20
Paravalvular Leak and Requirement for Postdilation
Conflicting evidence exists regarding the incidence of PVL in patients with and without pre-BAV. Some have hypothesised that direct implantation avoids disruption of the valvular calcium and allows the valve to sit more securely, thereby avoiding PVL, while others suggest that preparation of the calcium avoids non-circular valve expansion and underexpansion, along with the resulting residual PVL and higher valve gradients, which may be a particular issue with self-expanding valves. Although some studies have found a higher need for postdilation in direct implantation patients, others have found a less frequent need or no difference in postdilation frequency with direct TAVR.5,6,8,11,20 The DIRECTAVI trial using a balloon-expandable valve did not demonstrate any difference in rates of postdilation between those who had predilation versus those who did not.17 However, the converse was true in the DIRECT trial, again using the CoreValve system, in which postdilation was required more frequently in the direct implant arm (29.4% versus 15.1%, p=0.03).16 Neither study, however, demonstrated differences in aortic regurgitation at discharge between the groups, suggesting that postdilation can adequately resolve the issue of PVL.
General Recommendations on Predilation
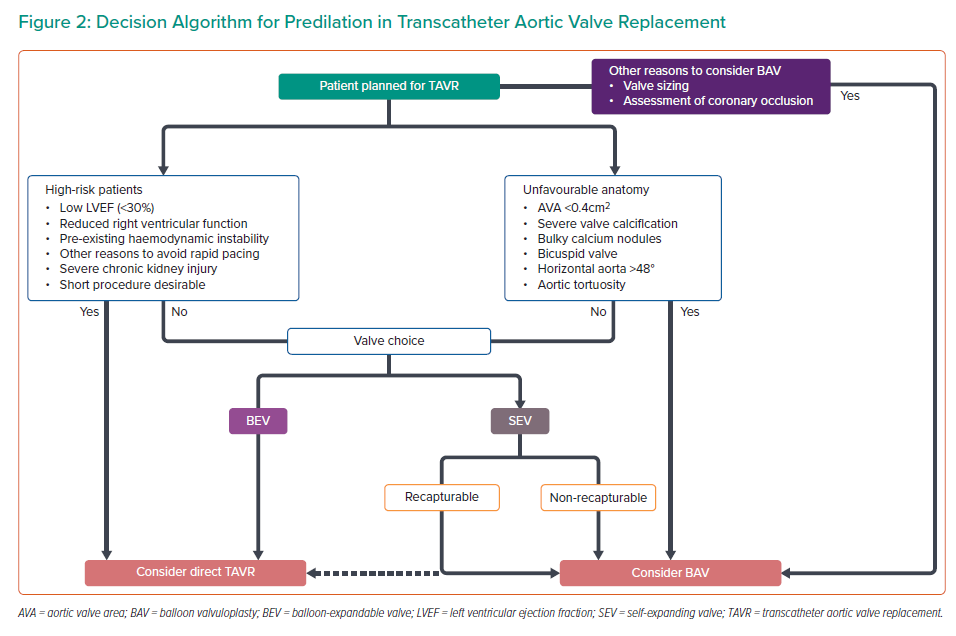
Although randomised data exist regarding the outcomes of direct TAVR versus predilation, recommendations for the specific clinical or anatomical scenarios in which predilation should be performed, are lacking. However, given that no consensus exists, this section will therefore detail the approach at our centre to performing predilation. The accompanying algorithm may provide some guidance to identify those patients in whom predilation is required and those who may be suitable for direct TAVR implantation (Figure 2). Our practice has been to consider three fundamental aspects: the clinical assessment of the patient, anatomical considerations, and the planned valve choice.
Clinical Assessment
Patients with impaired left or right ventricular systolic function may have a poorer tolerance of the haemodynamic shifts caused by rapid pacing during BAV. Our practice therefore has been to avoid predilation in patients vulnerable to the effects of hypotension such as those with reduced right or left ventricular function, severe chronic kidney disease, and those who may already be haemodynamically compromised, such as those undergoing rescue TAVR procedures. Additionally, patients who are unlikely to tolerate prolonged procedures or prolonged periods of sedation or intubation (severe pre-existing lung disease) are considered in our institution for direct TAVR implantation.
Anatomical Considerations
At our institution, multi-slice CT for valve sizing, access site choice and assessment of aortic tortuosity or angulation is performed for all patients, as is a thorough transthoracic echocardiogram. In this manner a thorough, anatomical assessment is performed to identify patients who will require pre-BAV. Islas et al. identified a number of echocardiography criteria that highlight patients for whom direct TAVR is unfavourable, including AVA <0.4 cm2, irregular valve orifice, presence of calcium nodules, and leaflet calcification greater than grade 2.19 Other studies have also reported on anatomical characteristics (Agatston calcium score and lower AVA) that have been associated with difficulties in crossing the stenosed valve.17,38,39 Predilation is therefore considered in heavily calcified valves (Agatston score >5,000) with severe stenosis at our institution. Excessive tortuosity or angulation of the aorta may lead to difficulties in crossing the native valve with the prosthesis. In self-expanding valves an aortic root angulation of >48° has been found to result in reduced device success with increased PVL. Excessive aortic root angulation may therefore be another consideration in the decision to perform BAV before TAVR.40
Congenital or acquired abnormalities of aortic valve morphology are also an important consideration. Bicuspid (either functional or ‘true’ type 0) aortic valve disease remains a complex anatomical subset, and studies on TAVR in these patients have used predilation in almost 100% of cases, which, until TAVR for bicuspid aortic valve disease comes into more widespread use, is likely to remain the recommended approach, and is our preferred approach.38,39 Balloon migration and asymmetrical elliptical stent frame expansion might be seen in patients with bicuspid valve and could be minimised with BAV. The 2017 American College of Cardiology consensus document for the use of TAVR in the management of severe aortic stenosis has also suggested that predilation may be useful if the coronary ostia are low-lying to assess the risk of coronary obstruction with valve implantation.41 In our experience, however, we do not use BAV for this purpose and elect instead to protect the coronary ostia by wiring with or without a stent prepared for deployment, depending on the risk and valve type.
Prosthesis Choice
Our final consideration is the prosthesis we plan to use. Self-expanding prostheses are known to have lower radial force and may be underexpanded or obtain a more eccentric geometry after deployment.42 Although evidence exists that the CoreValve family may be implanted without the use of predilation, we have a lower threshold for performing pre-BAV with CoreValve compared with balloon-expandable valves. Cases of infolding of the valve prosthesis have been reported with the CoreValve system and are thought to be related to eccentric calcification of the aortic annulus.43–46 Although postdilation can be used to correct infolding, predilation may be a more effective way of preventing valve infolding. Other self-expanding systems such as the Portico (Abbott Vascular) and ACURATE Neo devices (Boston Scientific) are almost always deployed after predilation at our institution. However, the ability to recapture the Portico device allows more flexibility if the prosthesis is severely underexpanded during the deployment process, in contrast to the ACURATE neo valve, which cannot be recaptured once the deployment process has begun. We consider it therefore even more important to perform predilation when using non-recapturable self-expandable valves. Our recommendation is to use a predilation semi-compliant balloon diameter equal to the minimum diameter of the aortic annulus for recapturable self-expanding valves, and a diameter 1–2 mm larger than the minimum diameter for non-recapturable self-expanding valves.
Postdilation
Optimal prosthesis frame expansion, reduction of PVL severity, improved effective orifice area and less patient–prosthesis mismatch have been noted after BPD of TAVR prostheses and are the main indications for BPD.47,48 In the OCEAN trial, the absence of balloon postdilation was associated with a 1.9-fold increased risk of patient–prosthesis mismatch on multivariable analysis.48 This is particularly important given that there is growing evidence that PPM may be a contributing factor to clinical and subclinical valve thrombosis.49 In the case of valve-in-valve TAVR, postdilation of the implanted valve with the aim of cracking the original surgical bioprosthesis and improving the effective orifice area of the TAVR, as well as improving the transvalvular gradients, has been recently adapted.50–52 However, paravalvular aortic regurgitation is the principal reason for performing BPD of TAVR valves.53 PVL occurs when there is incomplete apposition of the TAVR valve against the aortic wall.54 Avoidance of PVL remains one of the challenges of TAVR, given that the rate of PVL has been systematically higher following TAVR than with surgical aortic valve replacement in studies to date.55 However, newer devices have a much lower incidence of PVL, which is now commonly reported as being between 3% and 6%, and has clearly decreased over time.56–58
A number of predictors of PVL have been identified, including severe calcification of the aortic valve leaflets, large annulus dimensions, significant annular eccentricity, eccentricity index of the implanted prosthesis on CT, upper left ventricular outflow tract (LVOT) calcification, landing zone calcification, LVOT non-tubularity, aortic angulation >48° (in self-expandable devices), and lower device:annulus size ratio.37,40,42,53,59–65 Many of these risk factors can be identified on the pre-TAVR CT, and the appropriate prosthesis and prosthesis size to minimise PVL can be selected based on CT measurements, which has contributed to the decreasing rate of this complication and resulted in less frequent use of BPD.66,67
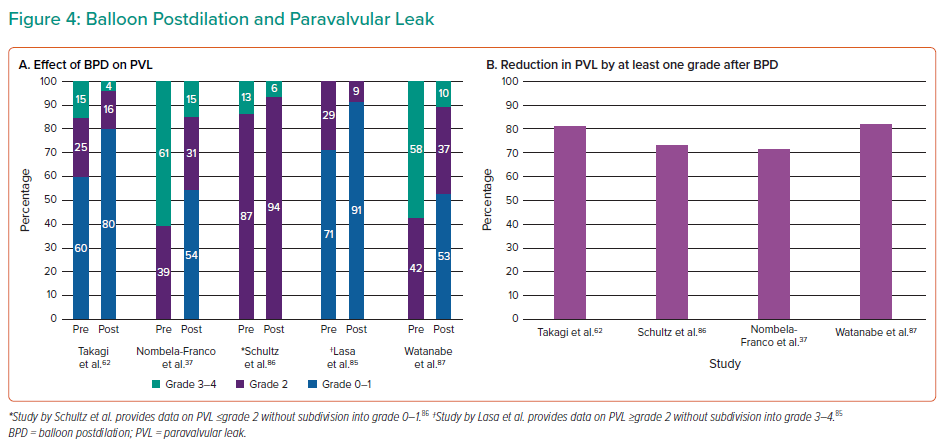
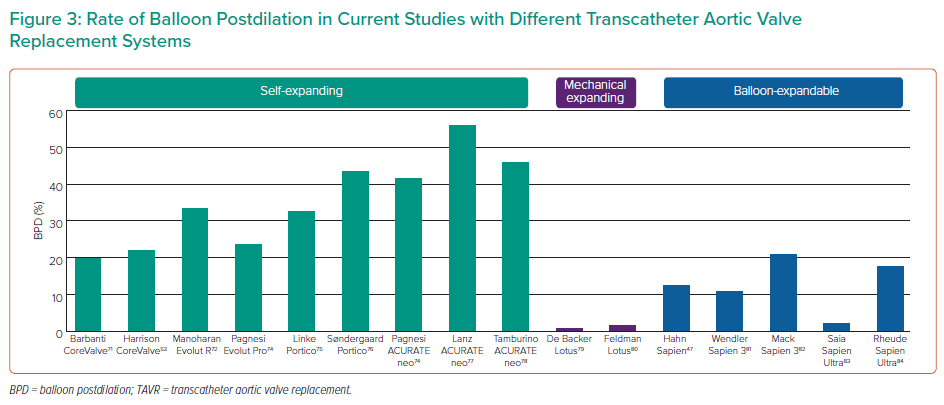
PVL, however, remains associated with poorer outcomes at follow-up, including mortality and readmissions, and therefore minimising PVL before the end of the procedure is important.68–72 Despite technological advances in TAVR prostheses, BPD remains a frequently performed adjunctive procedure even with newer generation valves (Figure 3).47,53,71,73–84 Its effectiveness in reducing the degree of PVL has been demonstrated in several studies (Figure 4A), with PVL reduced by at least one grade in ~70% of cases (Figure 4B).37,62,85–87 Therefore, BPD remains an important step for resolving PVL but it is not without its risks, which must be understood by those performing TAVR procedures. In the following paragraphs we will discuss the risks of BPD and some technical aspects of the BPD procedure.
Potential Risks of Postdilation
Aortic Annulus Rupture/Aortic Damage
Aortic annular rupture remains a thankfully uncommon complication (<1%), but it is potentially fatal and accounts for ~14% of surgical bailout procedures during TAVR.88 LVOT calcification and excessive valve oversizing (>20%) have been identified as risk factors.89,90 BPD has been identified as a cause of aortic annular rupture in a number of case series and studies.89,91,92 In a multicentre study by Barbanti et al. of 31 consecutive patients with annular rupture matched to 31 controls, balloon dilation had been undertaken in 22% of those with annular rupture versus 0% in the control group (p=0.005).89 However, no differences in major aortic complications and no episodes of aortic annular rupture were seen by Daneault et al., who studied patients included in the PARTNER and PARTNER 2 trials who underwent BPD.93 In an analysis of patients undergoing postdilation with the CoreValve system, Harrison et al. did not find any statistically significant differences in major vascular complications between those who did and did not require BPD, although three intraprocedural deaths occurred, due to annulus rupture, cardiac perforation and iatrogenic ventricular septal defect in the BPD group.53
Stroke
Cerebrovascular events have been linked to the requirement for BPD. Given that BPD is more commonly required in severely calcified valves and is supported by transcranial studies, dislodgement and embolisation of particulate matter during BPD is most likely the cause of these events.35 Nombela-Franco et al., in their study of 211 patients undergoing TAVR with a balloon-expandable valve, found an increased rate of acute cerebrovascular events in the first 24 hours in those requiring postdilation (8.5% versus 0.7%, p<0.007), with no difference between groups beyond 24 hours (postdilation versus no postdilation, 3.4% versus 1.3%, p=0.312).37 This was further explored in a multicentre registry study of 1,061 patients receiving both balloon-expandable and self-expandable valves, which again found BPD to be associated with cerebrovascular events, with an almost 2.5-fold increased risk in patients undergoing BPD.33 A 1.85-fold increased risk was also found in the EVERY-TAVI registry, again a registry of both balloon and self-expandable TAVR valves.94 Similarly, in a propensity score-matched analysis, Goel et al. demonstrated an almost fivefold increased risk of 30 day stroke or transient ischaemic attack in those requiring postdilation after a balloon-expandable TAVR (HR 4.95; 95% CI [1.02–24.03]; p=0.04), and analysis of those requiring BPD in the PARTNER trial also demonstrated an increased risk of stroke in the first 7 days after the procedure (for those requiring BPD versus no BPD, 4.9% versus 2.6%, p=0.04).47,95 Analysis of the CoreValve US clinical trials, however, did not find any difference in cerebrovascular events in those requiring BPD.53 Given the devastating consequences of stroke, minimising the need for postdilation by reducing PVL rates is of paramount importance. Accurate sizing of the annulus using multidetector CT has come some way in eliminating the need for postdilation of the implanted TAVR and should be advised for all cases of elective TAVR.
Conduction Disorders
Postdilation is another potential insult to the conduction system after valve deployment that could theoretically increase the risk of conduction disorders. However, the link between BPD and conduction disorders is weak and studies specifically evaluating this association are very limited. A tendency towards higher rates of LBBB in those requiring postdilation was found by Nombela-Franco et al., but with no differences in new PPM rates for patients receiving balloon-expandable Sapien valves.37 Analysis of the PARTNER and PARTNER 2 trials also did not find higher PPM rates in those requiring postdilation, nor did analysis of the CoreValve US clinical trials.53,93 However, Barbanti et al., in an analysis of 1,376 patients receiving CoreValve prosthesis, 19.8% of whom had undergone BPD, found a non-significant trend towards increased PPM requirement in those requiring BPD (29% versus 22.7%, p=0.092).71 Nonetheless, for patients with other risk factors for conduction disorders and pacemaker requirement, the risks of BPD must be balanced against the benefits.
Prosthesis Damage
Expansion of a TAVR prosthesis beyond its nominal size has the potential to cause damage to the pericardial leaflets, to the stent frame or, in the case of significant over-expansion, to lead to central aortic regurgitation. In a multicentre study by Armijo et al. of patients with large and extra-large annuli, the majority of whom required over-expansion of the TAVR prostheses either by addition of extra volume to the prosthesis balloon or by performing aggressive BPD, the incidence of central aortic regurgitation was 1.4%.96 During follow-up, postdilation was not associated with early valve degeneration or differences in valve gradients, however, there are no studies with long follow-up (>5 years) that specifically report the effects of BPD on valve haemodynamics. The potential damage to the prosthesis in the case of an aggressive BPD should be balanced against the potential positive effect of BPD in an underexpanded stent frame. In cases of significant underexpansion of the prosthesis, distortion of leaflet coaptation may have a negative impact on valve degeneration. Thus, BPD could be justified and have a beneficial effect on the prevention of valve deterioration.
Valve Embolisation
Valve embolisation can be a devastating complication of TAVR and is associated with higher mortality and major stroke at 30 days.97,98 A recent large multicentre study across 26 centres by Kim et al. found the incidence of valve embolisation to be 0.29%.98 Although malposition and device manipulation were the most common causes of embolisation, BPD was found to be the cause in 6.5% of cases of embolisation to the aorta and 3.6% of cases of ventricular embolisation.98 Makkar et al. also found postdilation to be one of the procedural causes of embolisation with the CoreValve prosthesis.97 The risk of embolisation must therefore be taken into account, especially if other risk factors exist such as suboptimal valve positioning.
General Recommendations on Postdilation
Given that no specific guidelines exist regarding postdilation, this section will outline the approach to BPD at our institution. The detrimental effects of PVL on outcomes have been clearly delineated.68–72 Our practice therefore is to perform both aortography and transthoracic echocardiography (trans-oesophageal only in complex anatomy) immediately after deployment of the device and, if valve position is correct, to perform BPD in all cases of grade III–IV PVL and to consider the risks and benefits of BPD in the context of grade II PVL. In younger, low-risk patients for whom optimal, durable results are vital, more aggressive postdilation approaches may be considered in the context of grade I–II PVL. In these scenarios, we perform BPD starting with semi-compliant balloons with a diameter equal to the mean annular diameter as measured on pre-TAVR CT and increase the balloon size according to the result. In the less frequent cases of high mean gradient (>20 mmHg) or frame underexpansion, we perform BPD to optimise the effective orifice area and reduce valve gradients. We usually start with a semi-compliant balloon size equal to the perimeter-derived diameter or mean aortic annulus diameter, and subsequently larger, if needed, after a careful assessment of the result. In the context of valve-in-valve procedures, we have adopted the approach of bioprosthesis fracture (if possible) with the aim of a mean gradient of <15–20 mmHg. In this context, non-compliant balloons are preferred with a size equal to, or slightly larger (~1 mm) than, the labelled diameter of the surgical bioprosthesis, and this has been shown to be effective in fracturing the bioprosthesis in a number of bench, and clinical studies.52,99–101 Our practice is to perform postdilation, as opposed to predilation for valve-in-valve TAVR. Although there are advantages and disadvantages to both, our experience suggests that there is less haemodynamic instability if postdilation and bioprosthesis fracture are performed in preference to predilation.
Conclusion
TAVR has revolutionised the treatment of symptomatic severe aortic stenosis with an exponential growing market. Successive iterations of TAVR valves have resulted in improved patient outcomes with comparable results to surgical aortic valve replacement even in lower risk patients. Valve durability becomes a major focus as we move into the low-risk patient profile group. As such, refinements in TAVR technique and optimisation of results are a priority. Although simplified TAVR procedures are now commonplace, this must not be at the expense of optimal results. As such, pre- and post-dilation of TAVR valves are likely to remain important adjunctive procedures for optimisation of the prosthesis and ensuring its durability. As outlined in this review, each has its place, with predilation facilitating native valve crossing with the prosthesis, ensuring uniform expansion of the prosthesis and reducing PVL; this comes at the expense of an increased risk of haemodynamic instability and conduction disorders. Postdilation too has a number of advantages, such as reducing PVL, correcting any frame underexpansion and optimising transvalvular gradients, but again there is a trade-off with the increased risk of stroke, valve embolisation or leaflet damage, conduction disorders and annular rupture. The risks and benefits as outlined in this review should be kept in mind when deciding in whom these procedures are required. With the huge strides in the TAVR field over the last decade, it is likely that the coming decade will also bring many changes to how TAVR is performed, highlighting the constant need to evaluate new data as they become available.