Ischaemic heart disease is a leading cause of heart failure with reduced ejection fraction (ischaemic cardiomyopathy) and confers a dismal prognosis, with a severe reduction in both life expectancy and quality of life, despite steady advances in heart failure medical therapy.1–3 The role of percutaneous revascularisation in managing these patients has been based on observational data and extrapolation from surgical trials.4–6 This has led to divergent clinical practice as clinicians struggle to reconcile the lack of randomised data with the pressing need for new therapies in this patient cohort.7,8 We review the rationale for revascularisation in ischaemic cardiomyopathy, with a focus on the recently published REVascularisation for Ischaemic VEntricular Dysfunction (REVIVED-BCIS2, hereafter REVIVED) trial, and how clinicians can integrate the results into their day-to-day practice.
Revascularisation and Mechanism of Benefit
The traditional rationale for coronary revascularisation in ischaemic cardiomyopathy is derived from the concept of reducing chronic ischaemia and restoring the function of hibernating myocardium. Myocardial hibernation represents a state of impaired myocardial and left ventricular (LV) function due to diminished coronary blood flow, which can be restored to a more normal state if the oxygen supply/demand relationship is improved by either an increase in blood flow or a reduction in oxygen consumption.9 It is an adaptive state to maintain myocyte viability in the setting of reduced blood flow.
Although the interactions between resting coronary blood flow, coronary flow reserve and myocardial function are debated, the purported benefits of revascularisation are multifaceted.
First, revascularisation of ischaemic myocardium improves metabolic function at the cellular level. Decreased myocardial blood flow leads to mitochondrial dysfunction and dysregulation of the sarcoplasmic reticulum, resulting in decreased adenosine triphosphate production and ion-pump impairment, which can lead to ischaemia; however, in hibernating myocardium, contractility is reduced proportionally in response to decreased blood flow, which interrupts progression to the final stage of necrosis.10 Restoration of normal myocardial blood flow leads to an improvement in metabolic function.11–13
In addition, revascularisation has the potential to reverse LV remodelling, leading to an improvement in LV volume and contractile function. Several functional features of hibernation have been identified to assess myocardial viability and aid in identifying patients who will experience an improvement in LV function. Because necrosis leads to thinning of the myocardial wall, a preserved end-diastolic wall thickness, measured by either echocardiography or cardiovascular magnetic resonance (CMR), may provide a simple method of assessing viability. Thinned myocardium at rest of ≤6 mm offers a <5% probability of LV function improving, whereas a wall thickness >6 mm predicts an improvement in function of >50%.14
However, resting wall thickness indicates only the status of the inner layer of myocardium, with the middle and outer layers contributing little to thickness. These layers respond to catecholamine stimulation and can thicken during dobutamine stimulation. Therefore, low-dose dobutamine stress echo (DSE) can be used and may indicate the presence of viable myocardium, which may attenuate LV remodelling.15 It is important to note that, in normal ventricles, there is significant variation in viable myocardial thickness, and a region of thinned myocardium may still have potential for recovery. Identification of the extent of scarring, using contrast-enhanced CMR, can also be used to determine the extent of myocardial viability. Regions of myocardium that have late gadolinium enhancement (LGE) indicate myocardial necrosis and irreversible injury.16–18
Similar to wall thickness, viable myocardium will show an improvement in contractile function, termed contractile reserve, with inotropic stimulation. Contractile reserve is determined by measurement of systolic function or LV dimensions, specifically change in LV ejection fraction (LVEF), wall motion score index, LV dimensions and LV global strain percentage change. The presence of contractile reserve has been shown to be a positive prognostic indicator.19 Radionuclide myocardial perfusion imaging, such as PET or single photon computerised tomography (SPECT), has also been used to assess viability. These tests measure preserved metabolic activity as demonstrated by myocardial uptake of labelled substrate in PET/SPECT; preserved sarcolemmal membrane integrity as demonstrated in delayed imaging in SPECT using thallium-201 or CMR; and preserved mitochondrial function as measured by technetium SPECT imaging.18,20,21
The recovery of dysfunctional segments as a mechanism for improved clinical outcomes such as heart failure symptoms and mortality has been suggested in observational studies and meta-analyses.20,22,23 However, the numerous tests available evaluate different facets of myocardial physiology and are only surrogate biological markers for true hibernating myocardium. The few randomised studies that have been carried out have all failed so far to show an interaction between myocardial viability and the treatment effect of revascularisation.24,25
Beyond Functional Recovery
Alternative mechanisms for benefit from revascularisation have been proposed. Given the leading cause of death in this population is sudden cardiac death (SCD) due to ventricular arrhythmia, revascularisation could potentially reduce the incidence of these arrhythmias. In acute or transient ischaemia, an increase in potassium creates injury currents between the normal and ischaemic tissue, which can initiate spontaneous activity. Re-entry, on the other hand, requires a trigger, such as a premature ventricular contraction, to trigger the arrhythmia and a substrate, such as scar tissue, to sustain it.
Following myocardial injury, fibroblasts remodel and develop abnormal electrophysiological properties in association with fibrosis. This can provide a vulnerable point for re-entry and create a pro-arrhythmogenic environment.26 CMR is the modality of choice for identifying scarring and fibrosis, and can be an effective tool in identifying both patients at a higher risk for SCD and those who could potentially benefit most from revascularisation.
Coronary Artery Bypass Grafting
A survival benefit with coronary artery bypass grafting (CABG) over medical therapy among patients with ischaemic cardiomyopathy was demonstrated as early as the 1970s through observational studies, such as the Duke 25-year Cardiovascular Disease Databank, although this was before the routine use of medications that are considered fundamental today, such as antiplatelets, statins, β-blockers and angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs).27
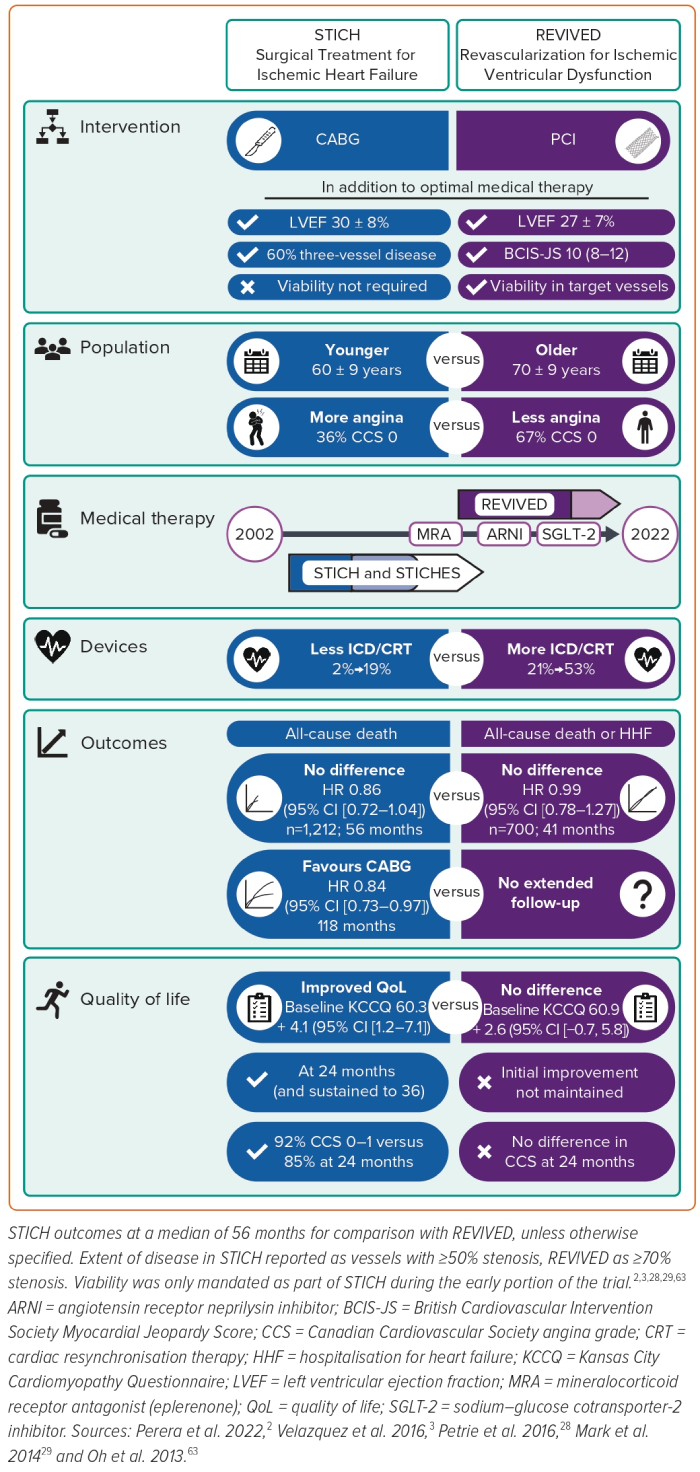
The more recent Surgical Treatment for Ischemic Heart Failure (STICH) study is the landmark trial providing the strongest evidence to support CABG among patients with LV dysfunction and coronary artery disease (CAD).3 STICH was a randomised parallel study that enrolled 1,212 patients with LV ejection fraction of ≤35% and CAD suitable for CABG, and evaluated outcomes with CABG plus medical therapy versus medical therapy alone. The primary outcome was all-cause mortality and long-term survival free of cardiac hospitalisation. Medical therapy included aspirin, ACE inhibitors/ARBs, β-blockers and statins. The CABG arm showed a threefold higher 30-day mortality but, by 5 years, all-cause mortality was similar between the two groups, with a reduction in cardiovascular death, MI and hospitalisation.
At 10-year follow-up, there was also a significant reduction in all-cause mortality with CABG.3 Further analysis of STICH demonstrated that the greatest all-cause mortality benefits manifested among younger patients.28 The mean age in the trial was 60 years and, while CABG had consistent effects on cardiovascular mortality irrespective of age, all-cause mortality and the combination of all-cause mortality and cardiovascular hospitalisation were reduced to a greater extent among younger patients in the trial.
A quality of life sub-study assessed the effect of randomised treatment on changes in health-related quality of life, measured by the Seattle Angina Questionnaire and changes in the Kansas City Cardiomyopathy Questionnaire (KCCQ). Treatment with CABG was associated with greater improvements in both metrics than medical therapy alone.29 However, despite 64% of the STICH population reporting angina at baseline (as assessed by Canadian Cardiovascular Society grade), the presence of angina was not a predictor of mortality or treatment effect in an additional post hoc analysis examining the relationship between anginal class and outcomes.30 These results conflict with previous findings that suggest angina is a marker of poorer prognosis and that there may be greater benefits from revascularisation in this group. However, this sub-study is limited by its retrospective design and, given there are no prospective trials comparing CABG versus medical therapy, external validation of its findings is not possible.
Another sub-study of STICH evaluated the impact of viability as assessed by SPECT and DSE on clinical outcomes. Viability testing was mandated only during the early phase of the trial; treatment allocation was not randomised according to its result; and viability was analysed in a binary fashion, with >11 viable segments as identified by SPECT defined as ‘extensive viable myocardium’ and less than this as ‘no viable myocardium’. Furthermore, the definition of viability by DSE was different, namely the presence of more than five dysfunctional segments with contractile reserve. Within these limitations, while patients with extensive viability appeared to significantly benefit from CABG at 10 years, statistical power was insufficient to test for an interaction between viability status and outcome.25,31
The role of viability testing to guide revascularisation in ischaemic cardiomyopathy in the wake of STICH remains contentious, and a more thoughtful approach may be required. It has been hypothesised that the survival benefit of CABG is due to the protection of remaining viable myocardium from future MI rather than functional recovery; therefore, identifying viable segments may still play a key role in planning surgical revascularisation.32,33
Percutaneous Coronary Intervention
CABG undoubtedly presents an early hazard to patients but, until recently, no prospective randomised study had evaluated whether percutaneous coronary intervention (PCI) could deliver the benefits demonstrated in STICH without this early risk. The REVIVED trial set out to test the hypothesis that PCI in addition to optimal medical therapy for heart failure would improve outcomes compared to optimal medical therapy alone.2
Using the concept of hibernation, PCI was given the best chance to succeed by requiring the presence of extensive myocardium at risk (with a British Cardiovascular Intervention Society Myocardial Jeopardy Score [BCIS-JS] of ≥6), with at least four dysfunctional but viable segments that were amenable to revascularisation. Over 6.5 years, 700 patients were randomised and, at a median of 41 months follow-up, the addition of PCI to optimal medical therapy showed no difference in the primary outcome of all-cause death or hospitalisation for heart failure (37% versus 38%; HR 0.99; 95% CI [0.78–1.27]; p=0.96).
REVIVED Population
The results of the trial should be considered in the context of the methodology. Equipoise required participants to not have an alternative indication for revascularisation. Patients with unacceptable angina were unlikely to have been randomised (two-thirds of those enrolled in REVIVED reported no angina at baseline). Conversely, we should also consider the potential masking of angina by poor functional status due to heart failure. By mandating the presence of viable myocardium and using the theory of hibernation, it is possible that a population with the cellular adaptation to avoid angina (at the cost of chronically reduced systolic function) was selected. Patients with recent acute coronary syndromes were additionally excluded by protocol, further contributing to the overall stability of the enrolled population. Finally, we can speculate that those young enough (with fewer competing risks of death) and fit enough for surgery to mitigate the early harm and realise the long-term reduction in cardiovascular mortality shown in STICH may have been offered CABG without consideration for enrolment in REVIVED.28 The mean age of patients randomised in REVIVED was 70 years.
Other REVIVED Outcomes
Beyond the headline result, this study has several other findings. A reduction in spontaneous MI was seen with PCI, which appears consistent with previous PCI trials in stable CAD with preserved LV systolic function, as well as the findings from STICH. Equally consistent is a failure of this to translate into a reduction in cardiovascular or all-cause mortality, at least in the short term. In this regard, REVIVED is not dissimilar to previous trials of revascularisation in people with stable ischaemic heart disease.34–36
A pre-specified secondary outcome was quality of life as measured by KCCQ. Although there was a greater improvement in KCCQ score with PCI in addition to optimal medical therapy (OMT) at 6 months, by 24 months there was no difference between the arms, as there had been improvements in the OMT arm alone. While the quality-of-life domain showed the same pattern of improvement with convergence of KCCQ scores between arms over time, this remained in favour of PCI at 24 months (4.2; 95% CI [0.4–8.1]). In interpreting these differences, the potential impact of crossover to revascularisation in the medical therapy arm of the trial, as well as the open-label nature of the trial, should be considered.
In addition, the rather modest improvement in overall LVEF was no different between the arms. We do not yet know how dysfunctional but viable myocardium responded to revascularisation at the segmental level but these analyses are under way.
ICDs (cardioverter-defibrillator as well as resynchronisation therapy) had been implanted in more than half (53%) of the population by the end of the trial, providing some insight into causes of death among patients with ischaemic cardiomyopathy and how this may be affected by PCI. All-cause death or aborted SCD occurred in 42% of the PCI group and 40% of the OMT group (HR 1.03; 95% CI [0.82–1.30]; p=0.80), with no difference in the occurrence of sustained ventricular arrhythmias or appropriate ICD therapies.37 It is well-established that ICD therapy continues to be underused in the contemporary heart failure population and, while the shortcomings of LVEF alone to predict risk of SCD in the ischaemic cardiomyopathy population are well known, alternative advanced techniques, such as scar quantification as well as characterisation by CMR-LGE or programmed ventricular stimulation, remain research tools for now.38–40 We can take away from REVIVED that ICD therapy should be strongly considered in all eligible ischaemic cardiomyopathy patients and not deferred until any revascularisation is performed.
Finally, a prespecified sub-study tested the impact of myocardial viability and, in contrast to STICH, this was considered as a continuous variable. When viability was quantified by the amount of myocardium that was dysfunctional yet viable, it was not associated with death or heart failure hospitalisation, nor with response to treatment assignment, nor with LV recovery.41 However, in the 70% of the patients who had undergone CMR (remaining 26% DSE; 4% PET/SPECT), scar volume was found to be a powerful predictor of outcomes. For every 10% in increase in scar burden, there was an increased risk of death or heart failure hospitalisation, independent of baseline LVEF (HR 1.18; 95% CI [1.04–1.33]; p<0.01). The reverse was not true for baseline LVEF; once corrected for scar, LVEF was not predictive of outcomes. Scar burden was also associated with likelihood of LV recovery (defined as greater than or equal to the median improvement of 4.7%; OR 0.69; 95% CI [0.56–0.84]).
These results complement the STICH viability sub-study and challenge how viability tests are used in revascularisation decision-making. PET was used in only a minority of patients, and so REVIVED cannot exclude the possibility that a metabolic assessment may be a better marker of true hibernation.
Perhaps a more intriguing area for future research is the potential for scar burden to be used to stratify risk and guide therapies, such as ICD implantation, even outside the confines of severe LV dysfunction.
STICH and REVIVED Compared
Two pivotal trials conducted over the last two decades have evaluated the role of revascularisation in ischaemic cardiomyopathy. It is tempting to summarise these trials as CABG works but PCI does not. There are some key differences between the trials that should also be considered (Figure 1). As we await further analyses from REVIVED, several early themes have emerged in the wake of the first publication.
How Sick Were the Patients in REVIVED?
REVIVED may have enrolled a population that was sicker than that in STICH, consistent with the population of patients who are often referred for PCI. The mean age of participants in the two trials differed by 10 years, which is remarkable, especially when compared across the two populations. If one hypothesises that the primary benefit of revascularisation on mortality is through prevention of adverse cardiovascular events, the rate of (off-target) non-cardiovascular events in an older population may have masked a potential treatment effect. Despite the high-risk profile of the patients enrolled, the medical therapy arm achieved comparable annualised event rates to the experimental arms of contemporary heart failure trials.42,43
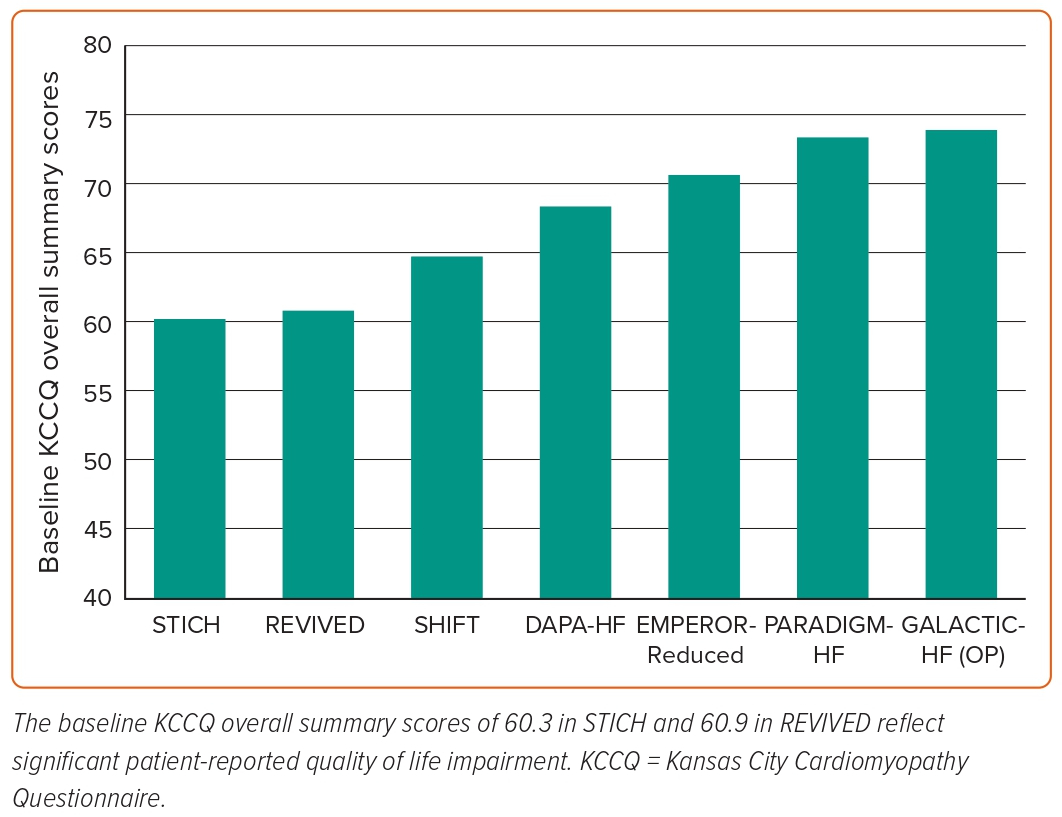
With respect to symptom status, the KCCQ score is a patient-reported measure of symptoms and function and is more reliable and sensitive than the New York Heart Association functional class.44 It is scored from 0 (very poor health) to 100 (excellent health), with an individual change of 5 points considered clinically significant by both clinicians and patients. There were identical overall summary scores in both arms of REVIVED at baseline (60.9, similar to STICH), indicating a significantly impaired quality of life, approximately 10 points worse than the baseline scores of contemporary heart failure trials (Figure 2).45,46 Median NT-pro-BNP within the PCI arm of REVIVED was also 11 times higher than guideline cut-off levels at baseline (1,376 pg/ml [697–3,426 pg/ml]); similarly, in STICH, BNP levels were nine times higher (313 pg/ml (190–569 pg/ml)).7,47
Enrolment with Non-ischaemic Cardiomyopathy
Using angiography alone, we can only make a probabilistic assessment that multivessel, proximal coronary disease causes severe LV dysfunction. Unlike STICH, REVIVED mandated viability testing throughout the trial. In this study, 70% (versus 21% of the whole STICH cohort) of patients underwent CMR as the method of assessment, and the power of LGE to distinguish ischaemic ventricular dysfunction versus coincidental coronary disease is apparent in the screening log.48 Of the 2,695 screened for eligibility, 326 were excluded due to the diagnosis of a non-ischaemic cardiomyopathy, despite fulfilling the inclusion criteria of extensive coronary disease.
Coronary Disease and Quality of Revascularisation
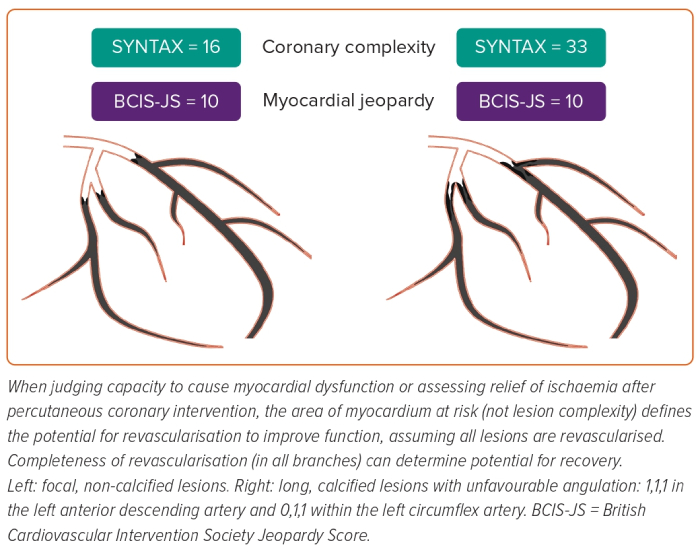
At present, analyses regarding the severity and amount of coronary artery disease in REVIVED (and completeness of revascularisation) are based on site-reported data. The most common structured measures of coronary disease and completeness of revascularisation are the SYNTAX and residual SYNTAX score, which weight both the location and complexity of the lesion (based on characteristics such as calcification, the presence of side branches and proximal segment tortuosity). However, ischaemic myocardial dysfunction is a function of flow restriction, rather than the complexity of the lesions per se (at a given anatomical location, a complex lesion will create similar jeopardy to a less complex one). Therefore, REVIVED used the BCIS-JS.49 This provides a simple, semi-quantitative assessment of the amount of myocardium at risk due to coronary disease. As a hypothetical example, the median BCIS-JS of 10 in REVIVED could represent significant proximal left anterior descending, major obtuse marginal and distal left circumflex artery disease within a left dominant circulation. The equivalent jeopardy (and capacity to cause ischaemic dysfunction in an equal amount of myocardium) could equally be provided with a low tertile SYNTAX score of 16, or a high tertile score of 33 (Figure 3).
The BCIS-JS applies a stricter requirement of ≥70% coronary stenosis (except for ≥50% in the left main) versus the ≥50% used to report the extent of coronary disease in the initial STICH manuscript. If relief of ischaemia responsible for myocardial dysfunction is the goal, it should be remembered that, of lesions visually assessed as 50–70% in severity, almost two-thirds will have an FFR >0.80.50 When a threshold of ≥75% visual stenosis was used across the whole STICH cohort (including surgical ventricular reconstruction), the proportion of patients with three-vessel disease was only 36%, with 23% having single vessel disease.51 While physiological significance is a comfortable approach for the interventionist, real-world practice of surgical revascularisation is anatomical rather than physiologically guided.52 Furthermore, protecting more myocardium with more distal anastomoses may ultimately be the mechanism for benefit of CABG in these patients.33 Finally, while complete revascularisation is assumed with CABG, this has not yet been characterised at the patient level in STICH. The results of the core lab-assessed extent of disease, quality of revascularisation and its impact on outcomes in REVIVED are eagerly awaited.
Was Treatment Comparable?
REVIVED ran from 2013 to 2020, with a recruitment rate similar to STICH, which began over a decade before. Over this time, there have been dramatic improvements in heart failure medical and device therapy (Figure 1). In 2002, the only class I recommendations for reducing mortality in symptomatic heart failure were ACE inhibitors, β-blockade and digitalis. Resynchronisation therapy and ICDs had no defined role.50 More recently, REVIVED has seen the introduction of angiotensin receptor neprilysin inhibitors followed by sodium–glucose cotransporter-2 inhibitors towards the tail end of its recruitment.42,43 If STICH were to be repeated in the current era of modern medical and device therapy, would a benefit still be seen with CABG?
Current Guidelines and Management
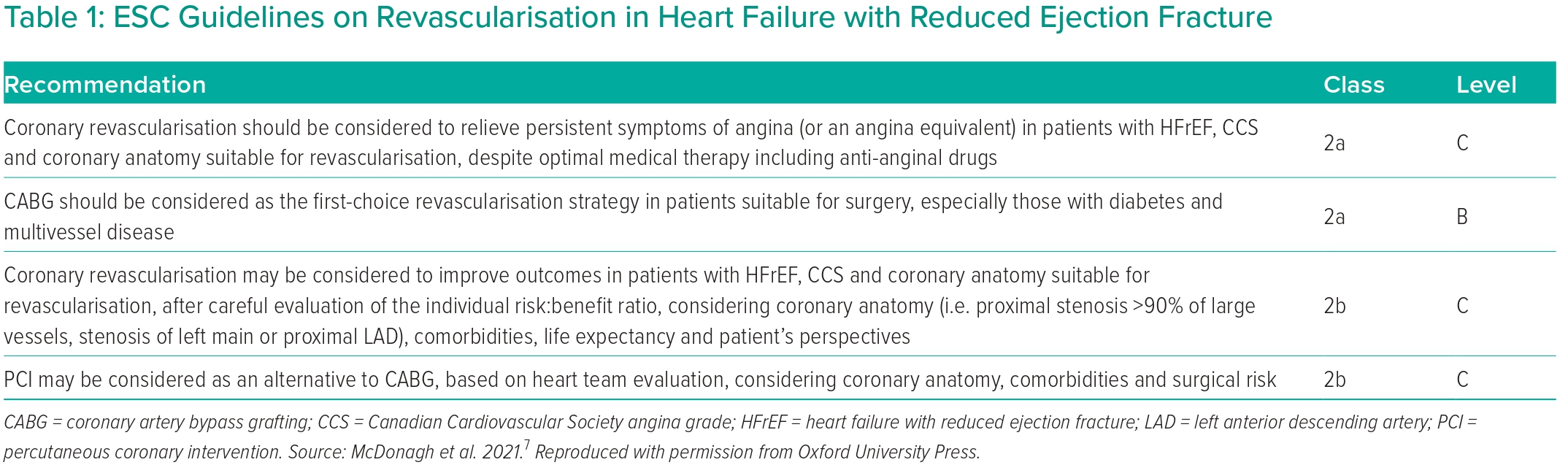
The conflicting data regarding the benefits of revascularisation in ischaemic cardiomyopathy are reflected in a discordance between the European Society of Cardiology (ESC) and American Heart Association/American College of Cardiology (AHA/ACC) guidelines. In 2021, the ESC downgraded its recommendations from 2018, which previously gave a 1b recommendation for revascularisation by CABG in severe LV dysfunction where the coronary anatomy was suitable, and a 2aC recommendation for PCI when complete revascularisation can be achieved, or on the recommendation of the heart team (Table 1).53 While the AHA/ACC/Heart Failure Society of America in 2022 continues to issue a strong recommendation for revascularisation by CABG, they recognise the lack of evidence to support PCI as an alternative to CABG.
How do we adapt our practice in light of the results of REVIVED? We suggest three areas to focus on (Figure 4).
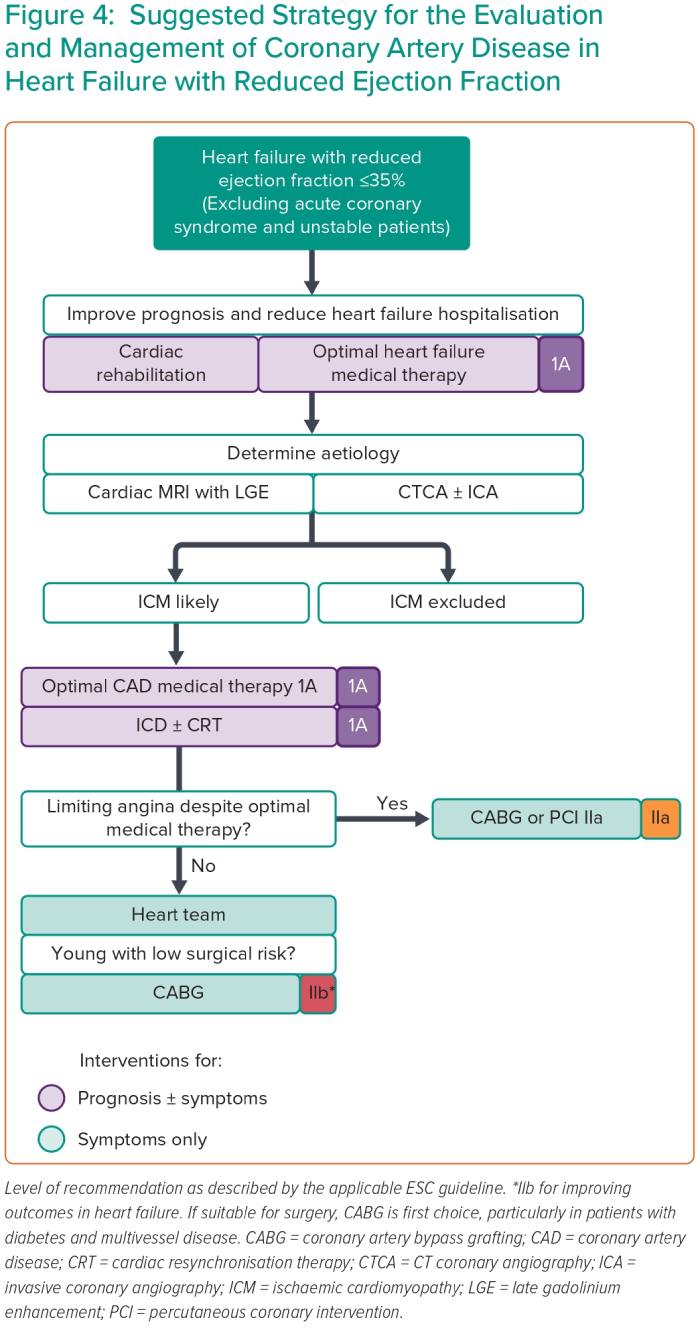
First, the diagnostic evaluation of the HFrEF patient remains relevant, especially as establishing an ischaemic aetiology has been historically underdiagnosed.54,55 Establishing an ischaemic aetiology of LV dysfunction delivers a strong mandate for an implantable cardiac defibrillator.7 A cardiac MRI with LGE can additionally provide prognostic information and distinguishes between ischaemic and non-ischaemic aetiologies.41,56–58 For those whose MRI suggests ischaemic dysfunction or provides no clear alternative diagnosis, an anatomical test should be performed. A non-invasive CT coronary angiogram (as local expertise allows) permits invasive angiography to be reserved for those in whom a CTCA is unable, or is unlikely to, deliver diagnostic information.
The second area of focus is the decision-making surrounding revascularisation of coronary disease in the presence of LV dysfunction. At present, a prognostic benefit from revascularisation appears to be derived only from carefully selected patients for whom bypass surgery is appropriate. Should patients be deemed prohibitively high risk for CABG, then optimising medical therapy in the first instance is appropriate.
PCI to flow-limiting lesions would seem to remain suitable for troublesome angina or symptoms that persist despite optimal medical therapy, although multimorbidity, multifactorial exercise limitation, as well as angina equivalents, can cloud assessment of the true symptomatic burden of coronary artery disease. However, evidence for PCI in the heart failure population is sparse, and CABG resulted in only an additional 9% of patients being rendered free of angina at a median time of 56 months in STICH over medical therapy alone (50% versus 41%).30
Third, and perhaps most important, is the crucial importance of guideline-directed medical and device therapy. Outside the context of randomised clinical trials, even after hospitalisation with newly diagnosed HFrEF, almost one in four patients will receive no prognostic medications at all.59 An improvement in delivering these fundamental and proven interventions must be a key take-home message. However, with death in almost one-third of the trial population at a median of 3.4 years, the prognosis in ischaemic cardiomyopathy remains far from bright.
Unanswered Questions
Given the severe LV systolic dysfunction and extensive myocardial jeopardy, PCI in REVIVED-type patients might be expected to carry a significant periprocedural risk, with a 30-day major adverse cardiovascular event rate of 40% and mortality of up to 6% in some series.60 Yet in REVIVED, the endpoint curves are superimposed. In other words, despite exposure to the known and significant risks of complex revascularisation, these patients accrue early events at the same rate as those in the medical therapy arm (and coincidentally, the same 30-day mortality rate as the medical therapy arm of the STICH trial). Mechanical circulatory support devices are proposed as potential solution in this context and both CHIP-BCIS3 (NCT05003817) and the PROTECT IV (NCT04763200) studies aim to address this. While it is possible that the periprocedural risk could be reduced further, the relative paucity of adverse periprocedural events in the PCI arm of the trial suggests that further reductions in events are unlikely to make this strategy safer in the periprocedural period.
Further elucidation of the relative roles of CABG and PCI in patients with ischaemic LV dysfunction is warranted. The mechanism of revascularisation with PCI, which is often applied as a focal treatment for a diffuse disease process, is fundamentally different from CABG. We can only speculate on the relative benefits of these modalities until long-term follow-up data are available or direct comparisons are made between CABG and PCI in patients with ischaemic cardiomyopathy who have a clinical indication for revascularisation.
Another unique aspect of REVIVED is the randomisation of patients with left main disease. The exclusion of this cohort from previous trials of revascularisation versus medical therapy (including STICH and ISCHEMIA36) reflects the belief that left main disease confers an unacceptably high risk of mortality, and that this risk can be effectively addressed by revascularisation. This is based upon observational data from the 1970s which suggests a 1-year mortality of up to 59% that is reduced with CABG.61,62 There appeared to be no difference in treatment effect in REVIVED depending on the presence of left main disease, although caution should be applied when interpreting sub-group data when the trial was not specifically designed to address this secondary hypothesis.
Future Research
Where does the current evidence for revascularisation in ischaemic cardiomyopathy leave us? With the first STICH patient having been randomised over 2 decades ago, how would CABG fare in the era of modern medical therapy and a high rate of implantable devices? The MASS-VI (HF) study (ISRCTN77449548) has completed recruitment, having been designed to recruit 600 patients with angina, multivessel disease and LVEF ≤35%, and randomise them to CABG or OMT. The primary outcome is a composite of all-cause death, non-fatal MI, stroke, and unstable angina requiring additional intervention at 5 years.
Given the difficulties in comparing STICH and REVIVED, it is difficult to consider how contemporary CABG would fare versus PCI. This question will be answered by the STICH 3.0 consortium, a proposed collection of seven independently funded, country-level trials (NCT05427370 and NCT05329285) that will directly compare PCI versus CABG in ischaemic left ventricular dysfunction. Results are not expected until 2029.
Conclusion
Outcomes in ischaemic cardiomyopathy remain poor. CABG in carefully selected patients may improve long-term outcomes but requires an individualised assessment of comorbidities and the likelihood of achieving complete revascularisation. It has become clear that PCI should not be performed solely for the purpose of improving LV function and reducing mortality among patients with HFrEF and stable ischaemic heart disease. Further insights from REVIVED into the interactions between ischaemia, viability, angiographic extent of disease and clinical outcomes are awaited.