Cell therapy, or the process of using cell-based treatments for the repair and restoration of function to damaged tissue, is quickly emerging as a new, cost-effective and personalised treatment modality that has the potential to address multiple unmet clinical needs, particularly in the field of cardiovascular disease. Present day interventions for the treatment of heart failure bears one of the highest cost burdens to the healthcare system due to invasive surgical procedures, lengthy hospital stays and high re-hospitalisation rates. The advent of cell therapy for the treatment of heart failure brings with it the potential for a minimally invasive, low-maintenance treatment option which may stabilise, or even reverse, ischaemic damage to the heart, while reducing long-term healthcare costs for heart failure patients, medical institutions and healthcare insurers.
Adipose Tissue as a Cell Source
Adipose tissue has begun attracting significant attention in the field of cardiac cell therapy as a source of regenerative cells due to its abundance within the body, ease of acquisition and high concentration of stem and regenerative cells. It has been shown to contain a unique combination of adult stem cells, endothelial and smooth muscle cells, tissue-resident macrophages and perivascular cells along with their progenitors, which in combination facilitate the body’s natural healing response to ischaemic environments.1 Collectively referred to as adipose-derived regenerative cells (ADRCs), this cell mixture has also demonstrated the ability to differentiate into spontaneously beating cells with cardiomyocyte features and secrete significant amounts of pro-angiogenic and anti-apoptotic factors to promote revascularisation and prevent cell death in an ischaemic environment.2,3
Another common source of mesenchymal stem cells being evaluated for cardiovascular applications is bone marrow. However, access to a therapeutic dose of bone-marrow derived stem cells requires ex vivo cell expansion, which is both time-consuming and costly. Adipose tissue has been shown to contain 100 to 1,000 times more pluripotent cells on a per cubic centimetre basis than bone marrow, suggesting that the cell culture required to expand stem cells from marrow may not be necessary when using adipose tissue as a cell source.4
Adipose-Derived Regenerative Cells for Cardiovascular Repair
A growing body of evidence suggests that ADRCs may be effective for the treatment of cardiovascular disease, which is among the leading causes of death around the world. Advancements in the treatment of acute cardiac events have been unable to prevent recurrent attacks, and compromised cardiac function post-infarction leaves this patient population more susceptible to developing refractory heart failure.5 With only a limited number of transplant organs available, implantation of ventricular assist devices remains the standard of care for end-stage heart failure patients. This procedure is highly invasive and complex in nature, and therefore results in prohibitive treatment costs and high rates of re-hospitalisation.
ADRC therapy, as a real-time treatment option to repair infarcted myocardium in the chronically failing heart has become a topic of much interest in the cardiovascular community. In particular, this therapy appeals to the portion of the heart failure population that are deemed non-revascularisable due to the presence of multiple co-morbidities or failure of conventional surgical or interventional techniques to be effective. These patients are generally classified as Class III–IV New York Heart Association (NYHA).6 ADRC therapy has the potential to be a new treatment option for this patient population and may prevent progression of the disease into end-stage heart failure and death. Further studies are needed to confirm this hypothesis.
Celution® – Adipose-Derived Regenerative Cells at the Point-of-care
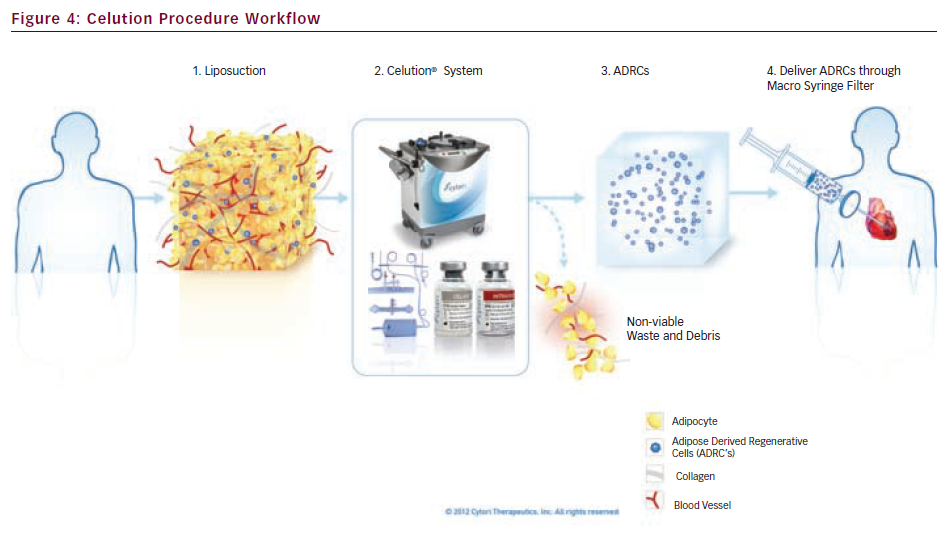
The Celution System (Cytori Therapeutics Inc., San Diego, US) was designed to safely and reliably extract autologous, medical-grade ADRCs at the patient’s bedside using clinically validated processing parameters and proprietary enzyme blends.6 The technology represents a significant advancement in cell therapy because of its ability to extract and deliver large numbers of diverse stem and regenerative cells in a single surgical procedure using a sterile, automated process.
The Celution System is currently the only Conformité Européenne (CE) marked medical device that processes adipose tissue to extract ADRCs at the point-of-care. It is currently approved for the treatment of multiple conditions such as breast reconstruction, soft tissue defects, Crohn's fistula, cryptoglandular fistula, and ulcers caused by radiation, ischaemia, or diabetes. It has a strong safety profile, with over 5,000 patients treated across various indications, including almost 50 patients treated in the cardiac setting.
The technology consists of four core components, collectively referred to as the Celution System:
- Cell processing device – the Celution device is an automated, electromechanical device containing multilingual, interactive software that facilitates the extraction and concentration of ADRCs, using extensively validated algorithms programmed into the device’s software. These algorithms optimise the safety profile and eliminate the potential for user variability by standardising the tissue disaggregation, centrifugation and therapeutic-specific formulation processes (see Figure 1).
- Cell processing consumable set – the Celution consumable set is a sterile, single-use set composed of multiple chambers. The consumable set is device-specific and provides users with a closed-system to process the tissue, eliminating the risk of microbial contamination during processing and subsequent patient infection (see Figure 2).
- Reagent – Celase® is an enzyme blend that releases ADRCs from the extracellular matrix of adipose tissue, and is optimised for use with the Celution device and consumable set. Celase is a sterile, pharmaceutical grade, mammalian-free reagent that is manufactured in compliance with Good Manufacturing Practices (GMP) standards (see Figure 3).
- Reagent – Intravase® is a secondary enzyme-based reagent blend used during Celution processing to optimise ADRC suspension for intravascular use. Intravase is a sterile, pharmaceutical grade, mammalian-free reagent that is manufactured per GMP standards (see Figure 3).
There are several techniques and devices available for delivery of cells into the heart. The appropriate approach is dependent upon disease state, morphology of cells and time of treatment, among other factors. For refractory heart failure, the optimal delivery technique is the transendocardial injection of cells into the hibernating myocardium. One technology that has been employed in several cardiac clinical trials, including Cytori’s PRECISE study, is the NOGASTAR® mapping and guiding catheter system (Biologics Delivery Systems, Diamond Bar, CA, US). Other systems include the Biocardia catheter, the Cardio 3 Bioscience delivery catheter and several others in development.
PRECISE – Clinical Safety and Feasibility
After conducting multiple cardiac-focused preclinical studies with ADRCs, two European, randomised, double-blind clinical trials using the Celution technology were conducted to study the safety and feasibility of ADRCs for acute myocardial infarction (APOLLO) and chronic myocardial ischaemia (PRECISE).
The PRECISE trial was the first clinical trial to evaluate the safety and feasibility of transendocardial delivery of ADRCs in patients diagnosed with chronic ischaemic cardiomyopathy that were refractory to other therapies. The trial was a prospective, randomised, placebo-controlled, double-blinded study that enrolled 27 patients, and utilised the NOGA System to deliver freshly isolated ADRCs into the ischaemic regions of the heart.
Inclusion criteria for the study was limited to patients with Class II to IV CCS angina symptoms and/or Class II to III NYHA symptoms of heart failure, left ventricular ejection fraction (LVEF) ≤45 % and evidence of a reversible stress-induced perfusion defect on single-photon emission computed tomography (SPECT) within one month of enrollment. Pre-screening of patients included imaging and other diagnostic tests to measure VO2 max, perfusion, arrhythmias and various other baseline indicators. Primary endpoints were limited to assessing the safety and feasibility of the procedure. Safety was measured by occurrence of major adverse cardiac or cerebral event (MACCE) and feasibility was measured by patients' tolerability of liposuction procedure and ability to inject ADRCs transendocardially.
After liposuction and ADRC or placebo injections, patients were followed for 36 months, with interim follow-up time points at six and 18 months. Follow-up results at both time points are summarised as follows:
- Local liposuction was well tolerated and ADRC preparation and transendocardial injections were feasible in all patients.
- Adverse events were similar in both sample groups, and none were related to the ADRC collection, processing, or cell delivery.
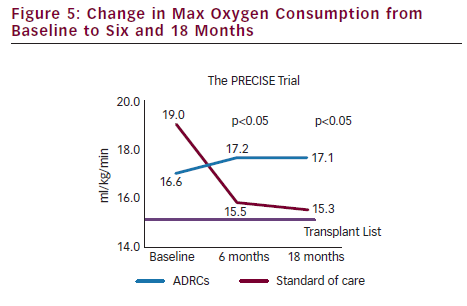
- VO2 max, a commonly accepted clinical indicator used to stratify patients potentially eligible for heart transplant, showed a statistically significant improvement from baseline to 18 months in the ADRC-treated group as compared to a decline in the placebo group. These results were observed at the six-month time point and maintained at the 18 month time point.
- Metabolic equivalents (METS), indicating a patient’s ability to perform physical activity, showed a statistically significant improvement from baseline to 18 months in the ADRC-treated group as compared to the placebo group.
- Left ventricular infarct size showed a decrease from baseline to 18 months in the ADRC-treated group as compared to the placebo group.
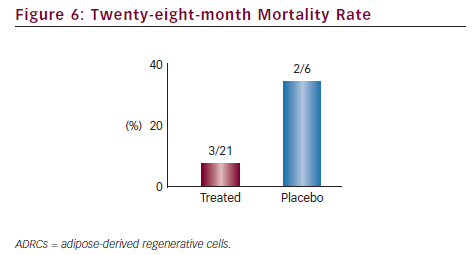
Results from the PRECISE trial successfully demonstrated that the collection, extraction, concentration and transendocardial delivery of ADRCs in the no-option chronic myocardial ischaemia patient population is safe and well tolerated. Additionally, while only a pilot study, the data also suggested that ADRC therapy may be an effective treatment for this disease indication, where conventional medial therapies have failed. The Celution System is also being evaluated in another pilot study, ATHENA, to treat the chronic myocardial ischaemia population in the US.
Conclusion
ADRC therapy using the Celution system has been shown to be safe and feasible for the transendocardial delivery in patients suffering from chronic ischaemic cardiomyopathy. The technology has demonstrated a strong safety profile on a broader scale with over 5,000 patients treated for various ischaemic conditions with minimal adverse events related to the ADRC procedure. The mechanisms of increased blood supply and immunomodulation are commonly seen in multiple wound healing applications, and suggest that the ADRCs may have the ability to repair damaged tissue in the chronic ischaemic heart. Early clinical data is consistent with this hypothesis, showing statistically significant improvement in VO2 max, a key predictor for potential eligibility for the transplant list, and positive trends in reducing mortality. If confirmed in larger studies, the Celution System holds the potential to offer the first autologous, cost-effective, point-of-care cell therapy option for patients suffering from refractory heart failure. It satisfies the two most critical criteria for cardiac cell therapy: autologous cells that avoid rejection and disease transmission risk, and point-of-care availability. While data is limited and evaluation of the therapy is still in its early stages, a growing body of evidence suggests that cell therapy in general, and ADRC-based cell therapy in particular, may evolve to be the next treatment paradigm to address the current unmet needs in cardiovascular disease care.