Mitral regurgitation (MR) is the most prevalent form of valvular heart disease in the developed world, affecting about 10% of people aged over 75 years.1–3 MR can be categorised as primary MR when it occurs as a result of pathological changes in the mitral valve (MV) apparatus and secondary MR when it occurs as a result of ventricular or atrial remodelling due to chronic volume overload leading to functional impairment with a structurally normal MV apparatus.4 The management of MR is dependent on numerous factors, including the aetiology, pathophysiology, natural history and expected efficacy of treatment. While the gold standard treatment of MR is MV repair or replacement, up to 50% of patients with severe symptomatic MR are not referred for surgery.5 Mortality rates for patients with severe symptomatic MR approach 50% at 5 years and the vast majority (90%) have at least one hospitalisation following diagnosis.6 Reasons for the low rates of surgical referral are multifactorial but principally relate to the presence of concomitant cardiac and non-cardiac comorbidities. Therefore, an unmet clinical need remains for the large subset of patients who have high risk of morbidity and mortality. Transcatheter MV replacement (TMVR) is a potential therapeutic option.
Over the past decade, several transcatheter MV repair devices have been used for patients who have a high or prohibitive surgical risk. These approaches have been derived from numerous surgical techniques. Transcatheter MV repair is most commonly performed using either the MitraClip (Abbott Vascular) device or the PASCAL system (Edwards Lifesciences). However, several other transcatheter MR repair approaches are in development or already undergoing clinical testing. The MitraClip and the PASCAL device mimic surgical edge-to-edge leaflet repair with the MitraClip being used in more than 100,000 procedures since its introduction in 2003 with high success and safety rates.7
Recently, the COAPT trial demonstrated that intervention with MitraClip in patients with moderate-to-severe or severe secondary MR and impaired left ventricular function resulted in lower all-cause mortality and lower rates of hospitalisation for heart failure compared with medical therapy alone at 24 months follow-up.8 On the other hand, the MITRA-FR trial did not demonstrate a benefit of transcatheter intervention compared with medical therapy.9
While several differences between these two trials have been discussed, including potentially lower operator experience and suboptimal optimisation of medical therapy in the MITRA-FR trial, the most widely referenced explanation for the disparate outcomes is the concept of disproportionately severe MR in combination with a more preserved left ventricle in the COAPT trial, hence leading to greater interventional efficacy.10 However, it is important to note that outcome data for the MitraClip is primarily based on treatment in patients with secondary MR.
TMVR is an alternative to transcatheter MV repair and offers several potential advantages. First, the pathophysiology of MV disease is complex, resulting in a heterogeneous anatomical spectrum that may be difficult to treat with current transcatheter MV repair devices. A TMVR device able to negate this heterogenicity and target numerous anatomical variations would therefore be a tangible advantage. TMVR would result in a standardised universal treatment for the treatment of MV disease, and it would result in more predictable reduction in MR than MV repair while remaining less invasive than current surgical techniques.11
Early experiences with TMVR have demonstrated that it is a feasible treatment option in patients who are at high or extreme risk for conventional MV surgery.12–16 In this review we provide a comprehensive overview of the feasibility and early clinical trial outcomes of TMVR devices that are currently under evaluation.
TMVR Devices
EVOQUE Transcatheter Mitral Valve
The EVOQUE transcatheter mitral valve (Edwards Lifesciences) is a self-expanding bovine tissue trileaflet prosthesis with a nitinol frame and polyester fabric skirt and a symmetric design that does not require rotational alignment to the mitral annulus.17 The frame features two sets of opposing anchors that secure and align the device with the native mitral annulus. The polyurethane foam-covered left ventricular anchors are designed to engage and preserve the subvalvular MV apparatus.17 The device can be delivered either via transfemoral/transseptal or transapical route using a 33 Fr delivery system.17
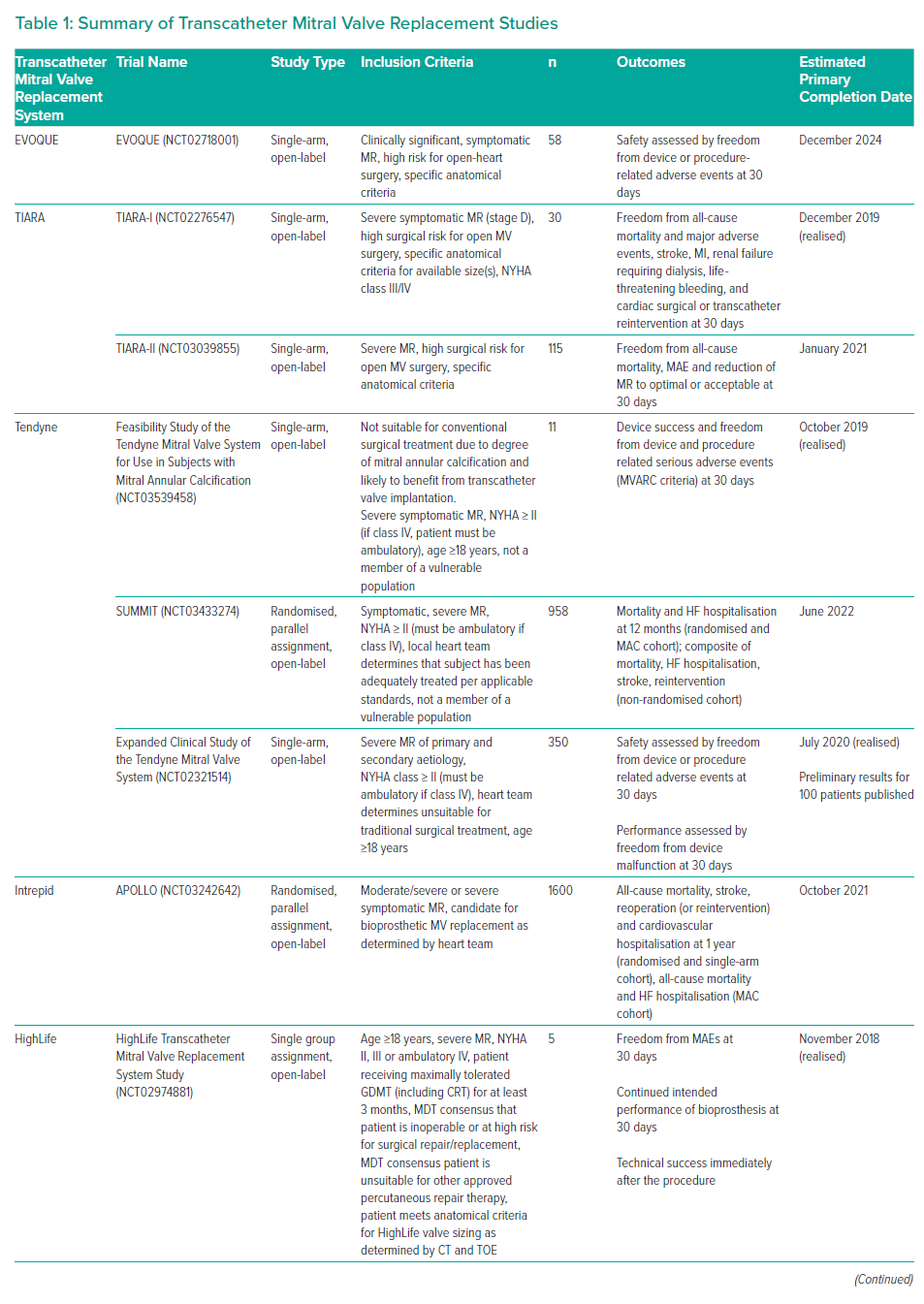
Numerous reports described early human experience with the CardiAQ valve (later reiterated to become the EVOQUE valve) following the first in-human implantation in 2012.18 Unfortunately, the RELIEF trial (NCT02722551) that aimed to evaluate the earlier CardiAQ valve was terminated prematurely due to safety concerns before recruiting any patients. Further design improvements resulted in the iterated EVOQUE valve with its own dedicated trial. The open-label single-arm transfemoral EVOQUE study (NCT02718001) is ongoing and aims to enrol 58 patients with a primary completion date of December 2024 (Table 1). The primary outcome measure is device and procedural safety and the secondary outcomes are New York Heart Association (NYHA) functional class, 6-minute walk test and reduction in MR grade.
Tiara Transcatheter Mitral Valve
The Tiara TMVR prosthesis (Neovasc) has a self-expanding nitinol alloy-based frame with a trileaflet bovine pericardial valve and can be delivered via a 32 Fr delivery system.19 The valve is D-shaped to geometrically fit the native MV annulus and the device is fixated radially by expansion of the Tiara valve and axially by the ventricular tabs (two anterior and one posterior).20 The ventricular tabs are designed to secure the valve (two attached to the anterior fibrous trigones and one anchoring the valve posteriorly to the shelf of the mitral annulus) thereby preventing migration and minimising the risk of paravalvular leakage, left ventricular outflow tract (LVOT) obstruction and coronary ostial encroachment.20 The Tiara TMVR has two sizes (35 mm: internal dimensions 30 × 35 mm, area 6.3–9 cm2; 40 mm: internal dimensions 34.2 × 40 mm, area 9–12 cm2) and is implanted via transapical approach.19
The first in-human implantation was performed in Canada in January 2014.19 The TIARA-I study (NCT02276547) is an ongoing international, multicentre, single-arm, prospective study of 30 patients, primarily examining 30-day safety outcomes in patients with severe MR at high risk for surgery. Secondary outcomes include performance, adverse events and clinical outcomes at 1 year.20 The TIARA-II study (NCT03039855) is an ongoing international, multicentre, single-arm, prospective study that aims to enrol 115 participants with an estimated completion date in January 2026. More than 50 patients have been treated with the TIARA TMVR with 95% implant success, no intraoperative mortality and 8.5% 30-day mortality.20 Given the valve design and mode of implantation, it will be challenging to implement the current design via transseptal approach.
Tendyne Transcatheter Mitral Valve
The Tendyne TMVR prosthesis (Abbott Vascular) is a fully repositionable and retrievable, double-frame designed device composed of a trileaflet porcine pericardial valve with an effective orifice area >3.2 cm2 mounted on a self-expanding nitinol frame and in January 2020 became the first TMVR device to receive CE mark approval.21 The inner circular frame of the prosthesis is of fixed size and supports the leaflets. The size of the outer (sealing) frame ranges from 30–43 mm in the septal-lateral dimension and 34–50 mm in the intercommissural dimension. The D-shaped prosthesis conforms to the shape of the MV apparatus with the straight edge resting on the atrial wall.21 It has a porcine pericardial covering and a polyethylene terephthalate cuff to assist mitral annular sealing. Anteriorly, the cuff of the outer frame extends above the annular plane, abutting the anterior atrial wall and aorto-mitral continuity.16
The device is one of the few TMVR devices to be completely repositionable and retrievable and it is delivered via a 34 Fr transapical sheath accessed via a small left anterior thoracotomy with a left ventricular apical tethering system and apical pad that anchors the device and assists with apical closure.21,22 The tension on the tether can be adjusted during the procedure to minimise paravalvular leak or LVOT obstruction.21
The first in-human implantation of the Tendyne prosthesis was in February 2013 and outcomes were reported the following year as part of a two-patient series demonstrating dramatic improvement in intracardiac pressures and grade of MR. Devices were then explanted and the patients proceeded to conventional valve replacement surgery as per the study protocol.23
The Tendyne Global Feasibility Trial (NCT02321514) is the largest published evaluation of this device and it looked at outcomes at 30 days and 1 year following TMVR with the Tendyne prosthesis via transapical delivery in a prospective non-randomised fashion.24 The trial enrolled 100 patients (mean age 75.4 ± 8.1 years, secondary MR n=89, primary MR n=11) at 24 study sites between November 2014 and November 2017. Successful device implantation was achieved in 96 patients (96%), with no intraprocedural deaths, two (2%) disabling strokes and two (2%) MIs during the hospital stay.
Mortality was 6% at 30 days and 26% at 1 year, with no MR in 98.4%, mean mitral gradient 3.0 ± 1.1 mmHg and no LVOT obstruction. Among survivors, 88.5% were in NYHA class I/II, with bleeding events and need for reintervention in 8% and 4%, respectively, at a mean follow-up of 13.7 months. Device-associated thrombus was observed in 6%, but no further thrombi were detected after a protocol change requiring post-procedural warfarin (target international normalized ratio 2.5–3.5) for over 3 months.24 The study is still expanding to gain further data on the safety and performance of the Tendyne device with the aim to include 350 participants across 40 centres over a 5-year period post implantation with an estimated study completion date of December 2025.
The SUMMIT trial (NCT03433274) is a prospective, multicentre clinical trial consisting of three arms: a randomised cohort (Tendyne versus MitraClip, 1:1 ratio), a non-randomised cohort treated with Tendyne and a mitral annular calcification cohort treated with Tendyne. The trial aims to enrol 958 patients with 1-year follow-up and completion is estimated in June 2026.
Intrepid Transcatheter Mitral Valve
The Intrepid TMVR system (Medtronic) consists of a circular trileaflet self-expanding bovine pericardial valve contained within a nitinol frame. It has a unique dual structure design, consisting of an inner stent with valve attached and an independent conformable outer fixation ring to engage mitral annular anatomy, accommodate the dynamic variability of the MV and mitral annulus and prevent disruption of the shape of the inner frame throughout the cardiac cycle.25 A flexible brim is attached to the atrial end of the fixation ring to facilitate imaging with ultrasound during implantation.13 The Intrepid TMVR system is built around a 27 mm inner valve structure with an effective orifice area (EOA) of 2.4 cm2 and outer diameters of 43 mm, 46 mm and 50 mm. Delivery is via transapical access guided by transoesophageal echocardiography (TOE) and fluoroscopy, and first implantations were undertaken in 2014.26
The APOLLO trial (NCT03242642) involving an estimated 1,600 patients started in 2017 and has two arms – one randomising TMVR versus traditional surgery in patients with severe, symptomatic MR and the second enrolling a single cohort of patients treated with TMVR who are ineligible for surgery. The primary endpoint is a composite of all-cause mortality, stroke, reoperation (or reintervention) and cardiovascular hospitalisation at 1 year with anticipated primary completion in 2021.
HighLife Transcatheter Mitral Valve
The HighLife device (HighLife SAS) is composed of two components – a subannular ring that is positioned around the native leaflets and a prosthetic TMVR that is positioned within. The sub-annular implant consists of a polymer tube with nitinol hooks for ring closure that is placed around the prosthesis to avoid displacement into the left ventricle and LVOT obstruction. Once in its final position, the native leaflets are trapped between the sub-annular implant and the prosthetic valve. The HighLife prosthesis is composed of a 31 mm nitinol frame and a trileaflet bovine pericardial valve with a pre-formed annular groove. Both the valve and ring are covered with Dacron and are completely endothelialised after a few months, embedding them to local structures and increasing stability.27 The prosthesis is available in one size and is delivered via transfemoral venous access and transseptal puncture, while the ring is delivered via transfemoral arterial approach.27,28
Initial results from a single centre feasibility study enrolling six patients treated with the HighLife TMVR were recently presented. Technical success was achieved in 83.3% (n=5) with one patient requiring conversion to open-heart surgery. There was one procedural mortality and a further mortality at 30-day follow-up, but no moderate or severe MR in the remaining four survivors.29 The HighLife Transcatheter Mitral Valve Replacement System study (NCT02974881) is a multicentre clinical study evaluating the feasibility, safety and performance of the HighLife TMVR system in patients with severe symptomatic MR who are unsuitable for surgical intervention and have heart team approval for percutaneous treatment. All patients will be followed up over 12 months with long-term performance and safety assessed annually up to 5 years after the intervention. Study completion is anticipated in December 2023.
Cardiovalve Transcatheter Mitral Valve
The Cardiovalve TMVR system (Cardiovalve) is a self-expandable trileaflet valve available in three sizes (range 40–50 mm) for delivery via transfemoral approach. It has a symmetrical design that is anchored into the mitral annulus by 24 atraumatic grasping legs. It has a low ventricular profile and protrudes into the left ventricle by only 12 mm after deployment. The Cardiovalve TMVR system has been successfully implanted into five patients with normal haemodynamic outcomes, no LVOT obstruction, no MR and either trace (n=3) or no paravalvular leak (n=2). Although three of the five patients died at 30 days due to issues with bleeding or vascular access, the surviving two have demonstrated promising outcomes, including sustained elimination of MR without further complications at 1 year.30
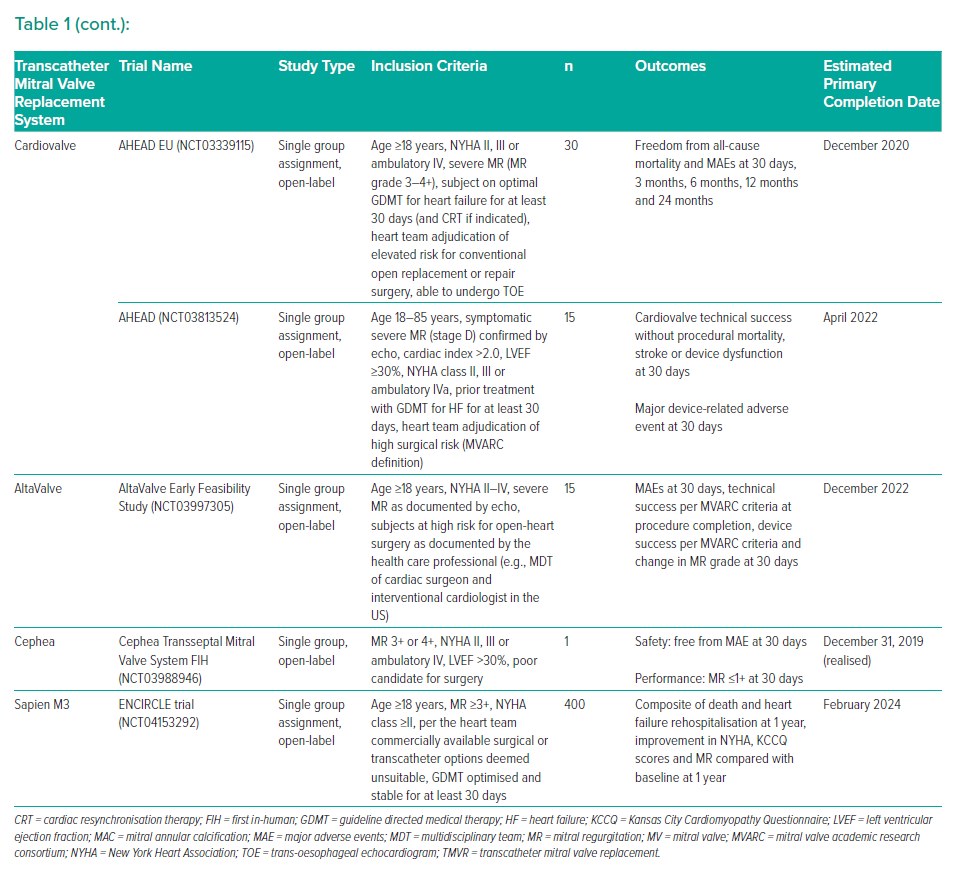
The AHEAD EU (NCT03339115) is a multi-centre, prospective, single-arm pilot clinical study enrolling a total of 30 subjects with severe MR requiring MV replacement and at high risk for open-heart surgery. The primary safety endpoint is freedom from all-cause mortality and major adverse events at 30 days. The US-based AHEAD trial (NCT03813524) is currently evaluating the safety and technical performance of the Cardiovalve TMVR system in 15 participants and is scheduled to complete in April 2027.
AltaValve Transcatheter Mitral Valve
The AltaValve device (4C Medical) is a repositionable, partially retrievable device that is implanted in a supra-annular position via transapical and transfemoral/transseptal routes. It consists of a self-expanding spherical nitinol frame (50–90 mm) that holds a 27 mm trileaflet bovine pericardial valve with an inferior fabric skirt to prevent paravalvular leaks.31 The first in-human implantation was in a 77-year-old man with high surgical risk and severe MR with a history of coronary artery bypass and surgical aortic valve replacement, reduced left ventricular ejection fraction (30%) and chronic AF. Preprocedural CT measurements were used to select a valve with a 70 mm frame and 46 mm annular ring with insertion via transapical approach and positioning under TOE guidance. Repeat CT and TOE imaging 6 days later revealed correct valve positioning and good apposition of the frame to the left atrial wall. The patient was discharged after 9 days with no adverse events at 30-day follow-up.31
The AltaValve is undergoing testing in an early feasibility study with estimated enrolment of 15 patients and planned primary completion in December 2022 (NCT03997305).
Cephea Transcatheter Mitral Valve
The Cephea TMVR system (Abbott Vascular) is designed for transseptal delivery and consists of a self-expanding double-disc stent structure carrying a trileaflet pericardial valve. The ventricular disc anchors the system in the sub-annular region and the atrial disc is deployed onto the base of the left atrium, fixating the valve through axial force without need for further sub-valve anchors. A multilevel conformability design enables adaptation to varying anatomy and a central decoupled core carrying the actual valve also prevents the leaflets from compressing the annulus. The low profile of the valve frame aims to prevent LVOT obstruction even in challenging anatomy.
Its first in-human implantation was reported in 2019 in an 83-year-old woman who had non-ischaemic congestive heart failure, AF and severe MR due to P3 prolapse with chordal rupture and a flail leaflet.32 Surgical risk was considered prohibitive and other percutaneous approaches carried a high risk of LVOT obstruction. Successful Cephea valve implantation resulted in immediate abolition of MR and significant improvement of heart failure symptoms (NYHA class 1) at 28-week follow-up with normal valve function, sustained left ventricular outflow and no intra- or paravalvular leak. The Cephea Transseptal Mitral Valve System FIH trial (NCT03988946) initially intended enrolment of 15 patients in an open label single-arm design, but recently completed enrolment after one patient – further information on additional trials is awaited. However, recently, early experience with the Cephea device in three patients has been reported in addition to the first in-human report.33 Implantation was successful in all patients with only mild paravalvular leak and no signs of LVOT obstruction or increased mitral gradients post procedure. After a median 6-month follow-up, valve function was sustained, echocardiographic parameters (mitral and LVOT gradients, paravalvular leak) remained favourable and all patients were in NYHA functional class II while experiencing improvement in quality of life according to the Kansas City Cardiomyopathy Questionnaire scores.
Sapien M3 Transcatheter Mitral Valve
The Sapien M3 transseptal TMVR system (Edwards Lifesciences) is based on the established S3 valve for transcatheter aortic valve replacement with a polyethylene terephthalate skirt knitted onto the 29 mm S3 frame for paravalvular sealing. A spiral-like nitinol dock featuring two wider turns (one capturing the native leaflets and one maintaining position in the left atrium) and several central functional turns to anchor the valve implant is initially deployed, followed by Sapien M3 valve delivery using the Edwards Commander system and implantation with balloon expansion.
Following publication of initial first in-human experience, 30-day outcomes of 35 high surgical risk patients with severe symptomatic MR treated within the ongoing single-arm US Early Feasibility Study of the Sapien M3 TMVR System (NCT03230747, planned enrolment of 50 patients) are now available.34,35 Technical success was achieved in 31 of 35 patients (one required paravalvular leak closure, one required separate transseptal punctures for dock and valve deployment and no valve was deployed in two patients). At 30 days, 1 patient (2.9%) had died and one had experienced a disabling stroke with a further case of valve thrombosis. Almost all (93.8%) patients had MR 0 or 1+ with a mean gradient of 5.6 ± 0.4 mmHg and 63.5% were in NYHA class I/II. While familiarity with the established Sapien valve platform (and proven durability in the aortic position) might offer advantages over other TMVR systems, complex interaction of the docking implant with the sub-valve apparatus could reduce ease of use.
The ENCIRCLE trial studying safety and effectiveness of the Sapien M3 device in 400 estimated patients just started enrolment (NCT04153292).
Early Transcatheter Mitral Valve Replacement Outcomes
Over 250 patients have now been treated with TMVR and recent pooled data of patients treated with seven different devices demonstrate that the vast majority had functional MR (76%), symptomatic advanced heart failure (NYHA class ≥III 80%, mean LVEF 42.6 ± 11.0%), and a high prevalence of AF (59%). Technical success was achieved in 92%, with conversion to surgery in 4%, LVOT obstruction in 4% and a procedural death rate of 2%. Pooled mean follow-up duration was 9.4 months with mortality of 13% at 30 days and 23% over subsequent follow-up, the vast majority due to cardiac causes. All surviving patients had MR grade <2 at follow-up with an observed device thrombosis rate of 5%.36
A recent systematic review by del Val et al. including data on 308 patients treated with TMVR confirmed the high technical success rate of 92% and reported a similar mortality at 30 days (14%) and after a mean follow-up of 10 months (28%).37 As currently only small early feasibility studies are available for most TMVR devices, it is not yet possible to robustly identify predictors of favourable clinical outcomes.
Assessing outcomes of the first 100 patients treated using the Tendyne system, the only TMVR device with larger clinical experience, Badhwar et al. identified prior percutaneous intervention (OR: 6.44, p=0.028) and renal insufficiency (OR: 8.15, p=0.022) as predictors of worse outcome after 1 year (evaluated using a combined endpoint of mortality and hospitalisation for heart failure), while preprocedural severe MR, when compared to moderate-severe MR, predicted freedom from the combined endpoint.38 The rate of LVOT obstruction in the first studies of TMVR has been very low, admittedly a result of rigorous preprocedural screening, hindering identification of anatomies increasingly prone to this complication.37 Imaging based models, incorporating factors such as device protrusion, flaring and angulation, aortomitral angulation, and a potential septal bulge, have been developed to assess the potential risk of LVOT obstruction, however, these concepts need to be validated in the clinical setting.39,40
The Future of Transcatheter Mitral Valve Replacement
The COAPT trial has established MV repair using the MitraClip device as the transcatheter treatment of choice for patients with high surgical risk and symptomatic severe MR.8 However, several limitations remain and the advent of TMVR has the potential to further transform the field of MV intervention. MV disease is complex and often multifactorial with a wide variety of disease patterns and specific anatomical criteria have to be met to ensure reproducibility of promising repair outcomes. TMVR devices may target numerous anatomical variations and negate this heterogeneity. In addition, TMVR requires highly experienced operators to guarantee optimal results whereas standardised treatment with TMVR may allow a more predictable reduction of MR. Furthermore, the long-term durability of transcatheter repair remains unknown – indeed, randomised surgical data have demonstrated significantly higher rates of recurrent MR following MV repair when compared to replacement (58.8% versus 3.8%).41 Thus, if repair techniques are used they should ideally allow for later valve replacement, which is only possible following MitraClip using electrosurgical laceration of the edge-to-edge bridge.42
Several challenges need to be addressed in the development of TMVR. Early generation TMVR devices (including Tendyne, the only system with CE mark approval), require large profile delivery systems and the majority remain limited to transapical access. Transcatheter aortic valve replacement demonstrated improved outcomes with transfemoral intervention and it is widely expected that there will be a significant reduction in periprocedural complications when transseptal TMVR delivery becomes feasible. In addition, large bulky devices have elevated risk of thrombus formation and specific pharmacological antithrombotic strategies are required after valve implantation.43 These factors – large profile delivery system with an increased risk of access-site bleeding and large devices with risk of thrombus formation – as well as the potential risk of afterload mismatch following TMVR with complete obliteration of MR still represent potential disadvantages of TMVR when compared to transcatheter repair approaches.
Furthermore, it is important to note that currently a considerable proportion of patients are not suitable for TMVR devices due to anatomical challenges, such as large annuli, the risk of LVOT obstruction and dimensions of the left ventricle. In line with these considerations, del Val et al. in their systematic review, when comparing main baseline patient characteristics and outcomes of the pooled TMVR population with those of the device group in the COAPT trial, found safety and procedure-related complications currently still favouring the transcatheter repair approach.37 Thus, existing and future TMVR systems will need to prove themselves in comparison with MitraClip, which has demonstrated substantial clinical benefits in terms of safety, mortality and need for hospitalisation.
TMVR is a challenging procedure requiring multidisciplinary collaboration, not only during evaluation of patients and preprocedural decision making, but also in safely conducting the procedure. Thus, it is essential that these procedures are performed in centres with established heart teams who have an established MV programme. The multidisciplinary heart team should at least be comprised of interventional cardiologists, cardiac surgeons, cardiac anaesthetists, cardiac imaging and heart failure specialists. Standardised treatment algorithms with regular multidisciplinary meetings using a wide range of treatment options for MV disease should be implemented.
Following the vast experience and widespread use of transcatheter MV repair (predominantly using the MitraClip device), TMVR, complementing the therapy armamentarium for MV disease, will probably be offered in the near to mid-term future to patients in whom sub-optimal outcomes of valve repair seem likely. Primarily, TMVR will be offered to patients for whom surgery is not possible due to age and/or comorbidities or in which transcatheter repair seems unfeasible due to unfavourable anatomical or functional parameters. Given the existing limitations of established MV repair technologies and the wide spectrum of MR patients and valve anatomy needing treatment, further development and evaluation of TMVR devices is necessary to enable tailored transcatheter treatment for individual patients.