In the early 1960s the first reports on successful aortocoronary bypass operations for the treatment of coronary artery disease (CAD) were published.1 Ever since, coronary artery bypass grafting (CABG) has become one of the most frequently performed operations worldwide and has been continuously refined and developed.2 Off-pump surgery and minimally invasive procedures have evolved to minimise the surgical trauma associated with CABG. In Germany, the unadjusted in-hospital survival rate for the 34,224 isolated CABG procedures was 97.3% in 2019.3
Calafiore et al. first described a left internal mammary artery (LIMA) to left anterior descending coronary artery (LAD) anastomosis via a small left anterior thoracotomy on the beating heart in 1996 and since then, minimally invasive CABG has been gaining wide acceptance in clinical practice, with many groups attempting to improve the procedure.2,4–9 The emergence of percutaneous coronary interventions (PCI) has notably intensified the search for less invasive procedures for surgical revascularisation. Despite the high rates of CAD, its optimal treatment is still the topic of ongoing debate. Both CABG and PCI have been subject to multilatitudinal scrutiny over the years.10–23 PCI is usually favoured due to its minimal invasiveness, especially in settings where patients can choose between these two modalities. Yet, multiarterial (MA) surgical revascularisation compared with PCI has resulted in substantially enhanced death rates and survival free of reintervention.24 Accordingly, MACABG represents the optimal therapy for multivessel coronary artery disease (MVCAD) and should be enthusiastically adopted by multidisciplinary heart teams as the best evidence-based therapy.24 However, one may not forget that the ART trial has shown that bilateral internal thoracic artery (BITA) grafting is not superior to single internal thoracic artery (SITA) grafting at least in the first decade following CABG.25,26 It may be possible that at further follow-up (at 15 or perhaps 20 years), a better survival of the BITA group may become apparent but until that time, SITA appears to be an equally good option.26
For the treating physician, factors such as predicted surgical mortality, the complexity of CAD as well as the anatomy and anticipated results filter into the decision-making process.27 The resulting risk–benefit ratio should be used to determine whether conservative therapy, PCI or CABG should be performed. Mohr et al. have focused on minimally invasive CABG and the implementation of robotic support using the da Vinci system (Intuitive Surgical).28–34 To overcome technical and anatomical limitations in totally endoscopic coronary artery bypass (TECAB), automated anastomotic devices to facilitate the procedure were developed.35–37 The innovative TECAB approach was performed in a number of cases with promising results.29 However, the original enthusiasm for this procedure was followed by a slow adoption rate on a larger scale. This occurred for several underlying reasons including the need to develop dedicated skills, the steep learning curve related to the procedure, the increased scrutiny of outcomes in CABG and the costs related to the robotic equipment.
Nowadays, minimally invasive direct coronary artery bypass (MIDCAB) grafting is the routine procedure for patients with isolated proximal LAD stenosis and also as part of a hybrid approach in selected patients with MVCAD.27,38 In general, avoidance of sternotomy and cardiopulmonary bypass (CPB) has allowed for faster recovery, resulted in less bleeding and fewer transfusions and helped to prevent wound infections.39 While MIDCAB initially mainly encompassed the revascularisation of the LAD with the LIMA, minimally invasive techniques are not restricted to patients with single-vessel disease, but can also be applied to selected cases of MVCAD.5,40
The use of both IMAs through a non-sternotomy approach was described by Balkhy et al. in 2017, using a totally robotic approach and recently by Davierwala et al. via a mini-thoracotomy incision. 41,42 In both cases, the sternal sparing technique enhances the adoption of both internal thoracic arteries as conduits and nullifies the risk of deep sternal wound infection, while providing the benefit of multiarterial bypass grafting.
This review discusses the available literature, describes standard approaches and elaborates on topics such as limited access procedures, indications and patient selection, diagnostics and imaging, different techniques, anastomotic devices, hybrid revascularisation, pitfalls and outcome analysis.
Methods
We searched the Medline database using subject and text terms for MIDCAB, TECAB, hybrid coronary revascularisation (HCR), robotic-assisted MIDCAB, anastomotic devices, fractional flow reserve (FFR), instantaneous wave-free ratio and PCI.
We limited our search to published review articles, case series and reports, retrospective comparative studies and randomised controlled trials (RCTs) between January 1998 and January 2021 to reflect contemporary practices regarding minimally invasive coronary revascularisation surgery in patients presenting with CAD. We also searched for meta-analyses in the above database and manually retrieved the most current meta-analyses that included RCTs, observational studies or both for the eight major topics. We also reviewed reference lists of identified studies. Duplicate references were identified and removed using the EndNote X5 Library (Thomson Reuters) program. Statistical software was not required because no numerical syntheses were performed.
Indications and Patient Selection
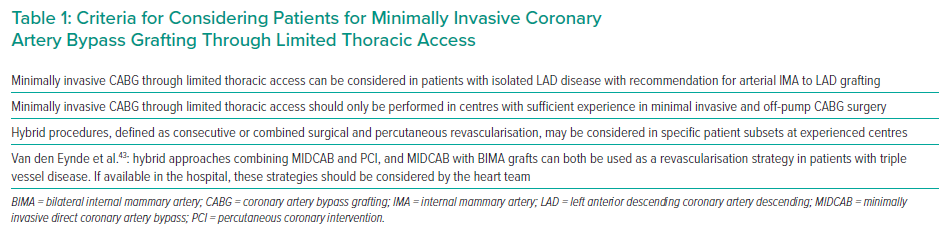
Heart teams are confronted with the challenge of incorporating minimally invasive strategies – off-pump CAB (OPCAB), MIDCAB, TECAB, PCI, and the hybrid approach – into the decision process, yet current guidelines do not fully address this challenge. The 2018 ECS/EACTS guidelines on myocardial revascularisation provide some criteria for the decision-making for minimally invasive and hybrid CABG procedures as shown in Table 1.27
In 2020, Van den Eynde et al. published a new decision tree that incorporates recent advances in minimally invasive revascularisation strategies, to optimise adequate delivery of care for each individual patient’s needs.44 In their decision tree, distinctions are made between single, double, and triple vessel disease and decision elements such as the SYNTAX-score, contraindication for dual antiplatelet therapy (DAPT), failed PCI and diabetes are added to guide the heart team’s decisions.44 Careful patient selection is of utmost importance to accomplish satisfactory minimally invasive coronary revascularisation. Regarding characteristics of the LAD itself, Diegeler et al. concluded that a diameter <1.5 mm, diffuse disease, severe calcification or intramural position of the LAD are exclusion criteria for MIDCAB.34 Second, they suggested that unfavourable anatomical conditions regarding sufficient exposure of the LAD and LIMA made MIDCAB unsuitable for women with obesity and/or large breasts and the authors recommended using a full sternotomy OPCAB approach in this patient group.34 While these recommendations reflect the early MIDCAB experience, in the current era only very few restrictions apply.
Amabile et al. reported that contraindications for the current TECAB practice were severe left pleural scarring (history of lung surgery or chronic granulomatous inflammatory processes), severe left ventricular dysfunction requiring the potential use of advanced myocardial support after surgery, and emergent cases.45
Minimally invasive surgical revascularisation has been found to be safe in single vessel disease as well as a selected group of patients with MVCAD, where it has been shown to have low complication rates, good long-term results and acceptable conversion rates.46,47 Furthermore, Al-Ruzzeh et al. found that patients had excellent subjective mid-term outcomes concerning their general health and quality of life.48 Nonetheless, it is important to keep in mind that any minimally invasive coronary operation remains more challenging than conventional CABG and that the choice of treatment strategy remains a controversial topic.
Techniques of Minimally Invasive Coronary Artery Bypass Grafting
The lack of a standardised nomenclature of different types of minimally invasive CABG procedures has generated confusion among cardiac surgeons and interventional cardiologists working in a heart team. In this manuscript we present the most adopted techniques and their variation to offer a general idea on how minimally invasive coronary revascularisation can be accomplished.
Minimally Invasive Direct Coronary Artery Bypass Grafting
MIDCAB grafting is currently the most standardised of all minimally invasive coronary procedures. It is performed through a small (mini) thoracotomy in the fourth left intercostal space (ICS) underneath the nipple. Surgeons perform both the LIMA takedown as well as the distal anastomosis of the LIMA to the LAD through this access. Grafting of mid-LAD and diagonal branches can be performed with this approach. The takedown of the LIMA can be challenging under direct vision, particularly in obese patients, women with large breasts or in tall patients with a long chest. Several surgeons have implemented the traditional MIDCAB technique with a videoscope inserted through a trocar in the second or third ICS to better visualise the proximal portion of the LIMA. This type of procedure is generally referred to as video-assisted MIDCAB. The use of a new retractor system and single-lung anaesthesia greatly aids in facilitating mammary artery exposure and dissection and allows a dedicated team to perform bilateral thoracic artery takedown from an anterior/antero-lateral thoracotomy using direct vision with video assistance.49
Robotic-assisted Minimally Invasive Direct Coronary Artery Bypass Grafting
Robotic-assisted MIDCAB refers to the combination of a robotic takedown of the left internal thoracic artery and a direct anastomosis of the LIMA to the LAD accomplished at the bedside of the patient by the surgeon through a small anterior thoracotomy. This procedure has become popular because it has several advantages with respect to the traditional MIDCAB procedure, where the LIMA is harvested through the anterior thoracotomy:
- By using the robotic platform, the visualisation of the LIMA is enhanced with lower risk of vessel injury and typically a longer IMA graft (until the distal bifurcation) can be harvested. If needed, the longer LIMA graft can be used to perform an additional sequential anastomosis on the diagonal coronary artery, thus enabling multivessel MIDCAB.
- During a traditional MIDCAB procedure, a special retractor is used to asymmetrically separate the two portions of the anterior thoracotomy and expose the mammary artery. Such traction is responsible for the postoperative pain experienced by most patients who undergo MIDCAB. By using the robotic platform to accomplish the LIMA takedown, the anterior thoracotomy performed in robotic MIDCAB is small and the retraction force applied to expose the LAD and accomplish the LIMA to LAD anastomosis under direct vision remains minimal which decreases the postoperative pain for the patient.
In robotic MIDCAB, the patient is positioned supine and a shoulder roll is applied on the left side of the spine to elevate the left hemithorax. Yet, some colleagues will not elevate the left chest as this may lead to pressure points. Single-lung ventilation is essential and the chest cavity is insufflated with CO2 to create additional space.
The camera port is placed about 3 cm lateral to the mid-clavicular line, usually in the fourth or fifth ICS, and two other ports are placed two ICS above and below this first port. The robotic arms are advanced to the bedside of the patient from the right side, crossing the midline, and are docked with the trocar previously inserted. The surgeon sits at the robotic console generally positioned within the operative room just on a side of the patient’s table and manouevres the two arms and the camera to accomplish the surgery. A table-side assistant (a second surgeon or a physician assistant) stands beside the patient and is in charge of exchanging the instruments as requested by the primary surgeon who sits at the surgical console. The mammary artery can be taken down both in skeletonised or pedicle technique using the robotic instruments.50 Side branches are clipped and cauterised. The entire length of the LIMA is mobilised. If required, both mammary arteries can be harvested by reaching the right internal thoracic artery with instruments still inserted using the trocars in the left chest and advanced through the midline crossing the anterior mediastinum into the right pleural space. The proximal portion of the right internal thoracic artery is harvested with the aid of the coronary stabiliser, which is inserted from a sub-costal trocar in the left chest and advanced to the proximal portion of the anterior mediastinum to expose the origin of the RIMA.41 After the IMA is harvested, the LAD is identified and finally a small anterior thoracotomy in the fourth and fifth ICS is performed to allow the surgeon to complete the LITA-to-LAD anastomosis at the bedside using specialised instruments and a coronary stabiliser designed for minimally invasive coronary surgery. Most of the centres adopting robotic MIDCAB grafting perform the anastomosis in an off-pump fashion on a beating heart. If two mammary arteries have been harvested, the anterior thoracotomy can be extended laterally and the second IMA deployed to the anterolateral (or lateral) coronary target.
As previously published by Van den Eynde et al., the extension of MIDCAB to vessels other than the LAD and its diagonal branches has in the past been hampered by two major challenges.43 First, in contrast to open surgery, manipulation of the heart through a thoracotomy is far more challenging.43 This has put an anatomical limitation to the extent of target vessels that can be reached, especially on the lateral and posterior sides of the heart.43 However, stabilisation devices that allow better exposure of the LAD as well as other vessels, have now become widely available.43 Second, the limited accessibility of the aorta during MIDCAB makes it difficult to perform proximal anastomoses for additional grafts.43 However, BIMA grafts combined with radial Y-grafts have been reported as an alternative to achieve revascularisation with multiple grafts.43
Une et al. evaluated the learning curve and its effect on minimally invasive cardiac surgery (MICS) CABG.51 They found that MICS CABG can be safely initiated without mortality or additional morbidity that could be explained by the learning curve. Pump assistance may be used without additional risk and represents a good strategy to avoid a steep learning curve and the possibility of conversion to sternotomy.51 Operative time reached an acceptable level at the 66th case in off-pump single-vessel small thoracotomy, the 16th case in CPB-assisted multivessel small thoracotomy (MVST) and the 40th case in off-pump MVST.51 Rodriguez et al. proved that in selected patients, MICS CABG can be safely initiated as a minimally invasive, multivessel alternative to open surgical coronary revascularization with excellent mid-term results.52 In their study, learning phase effects were not observed with regard to overall procedural safety, but rather in terms of improved freedom from bleeding, infection, conversion to sternotomy and repeat revascularisation.52
Totally Endoscopic Coronary Artery Bypass Grafting
The TECAB procedure was initially developed and performed to graft the LAD with the LIMA using the support of CPB in an arrested heart, as an even less invasive option than robotic-assisted MIDCAB. After demonstrating the safety and reproducibility of this approach in a case series, and through significant technological improvements of the following generations of the da Vinci robotic system, multiple conduit harvesting for more complex, off-pump grafting strategies became possible.53–55 It has been extensively demonstrated that robotic-assisted, endoscopic, multivessel CABG procedures are safe, feasible and reproducible and lead to excellent outcomes.6,8,56,57
In their propensity score matched analysis, Kofler et al. were able to demonstrate comparable perioperative and long-term results between highly selected robotic patients and conventional CABG patients, despite the longer operative times in robotic CABG.58 Moreover, several advantages of TECAB in comparison to any other strategy of revascularisation have been recently outlined.59 First, TECAB represents the paradigm of truly minimally invasive surgical myocardial revascularisation, being performed using five ports (8–12 mm) in the left chest, with no thoracotomy or sternotomy required. This lowers the risk of surgical site infection and minimises postoperative pain, allowing for a quick recovery, with early postoperative discharge in 2 or 3 days. Second, TECAB allows for multiple arterial grafting with the use of BITA with no risk of deep sternal wound infection even in high-risk patients. Moreover, in a closed-chest environment the right ITA is actually closer to the heart than generally perceived in an open sternotomy case and can reach left side targets passing underneath the anterior mediastinal fat. Thus, BITA can be used regardless of BMI, gender or glycated haemoglobin levels in patients with diabetes. Finally, an off-pump TECAB approach with BITA as conduits of choice provides all-arterial inflow which can be achieved in the left coronary system with a complete no-aortic touch technique, minimising the risk of stroke while offering the demonstrated benefit of a multiarterial revascularisation.60–62 Despite these numerous advantages, the penetration of TECAB is extremely limited due to the steep learning curve required to complete the distal anastomosis and properly stabilise the coronary target using the robotic platform. For these reasons, anastomotic devices need to be developed to facilitate TECAB.
Kofler et al. were able to demonstrate both minimally invasive procedures (MIDCAB versus TECAB) as feasible and safe, regarding perioperative clinical outcome. No perioperative death occurred and they observed an MI rate of 1.5% versus 0% (p=0.463) and a stroke rate of 1.5% versus 0% (p=0.454) in TECAB compared with MIDCAB, respectively.63 Their results were in line with previously published literature.
Stastny et al. proved that arrested heart TECAB resulted in excellent clinical long-term outcomes with a LIMA artery patency rate comparable with conventional CABG at 10 years after surgery.64
Anastomotic Devices
One of the first CABG procedures ever performed was done on the beating heart using an anastomotic device.35 In 1960, Robert Goetz used a tantalum Payr’s cannula to construct an end-to-end anastomosis between the right LIMA and the right coronary artery (RCA), demonstrating the feasibility of performing arterial grafting on the beating heart.35,65
The ideal anastomotic device should be easy to use, produce a geometrically optimal reproducible anastomosis with minimal endothelial damage and minimal blood-exposed non-intimal surface, yet a number of design constraints apply.36,66,67 Data directly comparing device and hand-sewn anastomosis in minimally invasive CABG remains very limited.68,69 The majority of available data exists for the C-Port Flex-A anastomotic device (Aesculap), which is the only device supported by extensive clinical data on its safety and patency published by several teams in Europe and the US.70 This remains the only distal anastomotic device that has been cleared by the FDA. The C-Port connector is a single-shot anastomotic device which completes the coronary anastomosis with an interrupted row of 13 microscopic stainless steel staples. A recent histological study showed it to be comparable to a hand-sewn coronary anastomosis regarding inflammatory response development of neointimal hyperplasia.71,72 Thus, further adequately powered Phase IV clinical trials are needed to compare anastomotic devices to hand-sewn techniques with carefully selected patient groups, considering factors such as target coronary vessel territory, calcification and quality as well as the choice of conduit. The results should detail morbidity and mortality outcomes, particularly focusing on combined major adverse cardiac and cerebrovascular events (MACCE) in the short and long term.66
Unfortunately, Aesculap suspended the production of this device after the technology was purchased from the original manufacturer with no official intention to bring it back on the market. This decision has been a massive step backwards for minimally invasive coronary surgery and reflects the lack of industry support in this field. There are only two other devices in pre-clinical development: the S² Distal Anastomotic System (iiTech) and the ELANA system (AMT Medical). A first-in-human clinical trial is imminent for the latter.
Hybrid Coronary Revascularisation
The rationale for hybrid coronary revascularisation (HCR) lies in the well-established survival benefit conferred by LIMA-to-LAD grafts and the use of new stent platforms featuring lower stent restenosis and thrombosis rates compared with venous graft stenosis and occlusion rates, respectively.73
When comparing CABG to PCI, CABG remains the gold standard in MVCAD, with lower mortality and lower repeat vascularisation risks. Despite the higher stroke risk suggested by CABG, that risk does not outweigh its benefits in long-term survival, leading physicians to combine the two procedures in what is known as HCR. Here, both surgical bypass and PCI are encompassed in that they are either performed during the same procedure or within 60 days of each other. Repossini et al. concluded that HCR is a safe approach with acceptable long-term results and that it could be offered to high-risk patients and to MVD patients whose non-LAD lesions, after careful evaluation, were judged more suitable for PCI than for CABG. Considerable experience with MIDCAB and close cooperation between surgeons and cardiologists are mandatory to ensure the optimal revascularisation strategy is decided for each individual.74
In general, there are three different approaches to HCR:
- The hybrid approach: LIMA–to-LAD surgery via a non-sternotomy approach (MIDCAB, robotic MIDCAB or TECAB), followed by PCI stenting of the non-LAD territory. The latter is generally performed 30 days after minimally invasive LIMA-to-LAD surgery.
- The reverse hybrid approach: in this scenario, PCI stenting is performed prior to minimally invasive CABG to the anterior ventricular wall or to the left coronary artery. The reverse hybrid approach generally happens in the light of an acute coronary syndrome involving a non-LAD target receiving acute PCI and consequent stent implantation. During emergency stenting, a diagnostic catheterisation of the left coronary system is accomplished yet additional stable CAD is noted. At this point, the surgeon is consulted to complete the revascularisation, applying a minimally invasive approach for LIMA-to-LAD surgery several weeks after the primary acute PCI. In this case, awareness should be raised regarding the need of DAPT. Nevertheless, the risk of bleeding during surgery should be addressed appropriately.
- The advanced hybrid procedure: this refers to any type of HCR that combines minimally invasive, sternal sparing, multiarterial bypass grafting with PCI.75,76 The standard hybrid approach only uses the LIMA to bypass the LAD. The advanced hybrid approach uses both mammary arteries for deployment to the left coronary system (LAD and circumflex) whereas the RCA is treated with a stent some weeks after the initial minimally invasive surgical procedure. The advanced hybrid procedure is also adopted when a single mammary artery is used in a minimally invasive way to bypass the LAD and diagonal coronary artery sequentially, followed by PCI of another non-LAD target (obtuse marginal or RCA).
The advantages and disadvantages of one stage (simultaneous) and two-stage HCR were described and published in 2015 by Panoulas et al.73
The results from the POLMIDES trial showed that HCR is feasible in selected patients with MVCAD referred for conventional CABG.77–79 Foik et al. concluded that patients receiving the HCR treatment required administration of pressor amines less frequently and less often experienced hypotonia compared with the group receiving the classic treatment (conventional CABG or OPCAB).78 On the other hand, oxygen saturation was significantly lower in the HCR group compared with the group receiving the classic treatment. Mobilisation of patients in the two-stage regimen of hybrid treatment was slower during the first two days and during cycles of rehabilitation but these patients achieved full self-reliance earlier than those from the classic group. Observational data on HCR from a multicentre study suggested that there is no significant difference in MACCE rates over 12 months between patients treated with multivessel PCI or HCR.80
The findings of Modrau et al. suggest non-superior 3-year clinical outcome after HCR compared to conventional myocardial revascularisation. Consideration of the procedure-associated morbidity may assist the heart team to provide an individualised revascularisation strategy.81
Various studies have shown that HCR resulted in fewer blood transfusions, shorter hospital stay, decreased ventilation times and shorter time for patients to return to work when compared to CABG, whereas CABG was more cost effective overall.82 The greater costs of HCR could be due to the use of radiographic instruments and stent implantation; however, it is suggested that with increasing experience these costs could be lowered. Both Reynolds et al. and Leacche et al. found that MACCE was significantly worse with HCR in high-risk patients, however in the mid-term (18 months), no difference in MACCE between the HCR and conventional CABG group was found.82,83 In fact, HCR patients showed lower stroke rates at 30 months.38,83,84
In their large series of HCR and multivessel PCI for patients with left main stenosis, Repossini et al. demonstrated favourable outcomes for HCR for patients with a medium–high EuroSCORE and a SYNTAX score <32, HCR may provide a promising alternative to conventional CABG and multiple PCI with similar postoperative results.85
HCR presents an attractive alternative option for treating patients with MVCAD because it maximises the clear survival benefits of LIMA-LAD grafting, improves quality assurance with completion angiography and allows quicker patient recovery; furthermore, patients avoid the negative systemic inflammatory effects of CPB and delayed healing after sternotomy.38
HCR most commonly involves a planned combination of LIMA-LAD grafting and PCI of non-LAD targets.86 One-third of US hospitals with on-site cardiac surgery perform HCR where it is reserved for a highly selected population.86 Clinical outcomes after HCR appear favourable, with lower MACCE, MI and repeat revascularisation rates compared with multivessel PCI.86 It has also been linked to lower stroke rates and in-hospital complications compared with CABG, but a greater need for repeat revascularisation.86 Engagement from interventional and surgical communities and adequate patient selection based on local expertise and data from registries and RCTs are of key importance to determine its future success.86 In light of data showing that drug-eluting stents are equivalent if not better than saphenous vein grafts, should we not be pushing the envelope and greatly expanding our use of HCR at the expense of traditional LIMA–LAD + saphenous vein graft CABG?88 The use of bilateral internal thoracic grafts improves overall long-term survival and repeat revascularisation-free survival without increasing the incidence of operative complications, including deep sternal wound infection, especially with the addition of graft skeletonisation.79,88,89 The short- and mid-term endpoints in this study would be unlikely to tease out these differences, so perhaps we should be more aggressive with BIMA grafting in any patient undergoing surgical myocardial revascularisation, including those who fit the ideal two-vessel HCR case and not just the young and relatively healthy.88 A hybrid approach could also be applied in an acute setting (reverse HCR) – treating the culprit lesion with PCI and then completing the revascularisation of other targets with minimally invasive CABG.
There are many limitations of HCR. For one, the operation is challenging as surgeons have to work through small incisions. This makes it particularly difficult for inexperienced doctors as good results strongly depend on the quality of the anastomosis. Furthermore, there are no gold standard criteria for patient selection.90 Esteves et al. showed with the long-term follow-up of the randomised MERGING clinical trial that HCR was feasible but associated with increasing rates of MACCE during 2 years of clinical follow-up, while the control group treated with conventional surgery presented with low rates of complications during the same period. They postulated that, before more definitive data arise, HCR should be applied with careful attention in practice, following a selective case-by-case indication.91 Recent studies have fostered the opinion that CABG remains the gold standard in patients with MVCAD; however hybrid approaches are an attractive option for certain patient groups.73 Current evidence suggests that HCR is feasible and safe for a particular target group with acceptable mid-term outcomes that are non-inferior to conventional CABG: just over 60 years of age; mainly stable, CAD-favourable anatomy; intermediate risk and SYNTAX scores; and preserved or mildly impaired left ventricular ejection fraction.73 However, data for higher-risk groups, who would theoretically benefit the most from HCR, are weak or lacking; hence, no inferences or generalisations can be made regarding the role of HCR in these patients.73 The 2012 American College of Cardiology and American Heart Association guidelines recommend HCR in patients with heavily calcified proximal aortas, inadequate bypass conduits and landing targets for non-LAD vessels that are feasible for PCI.92 Furthermore, the 2018 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularisation highlight the need for multicentre studies to prove the efficacy and superiority of hybrid techniques in stable MVCAD.27
Nonetheless, Ganyukov et al. proved that inpatients with MVCAD amenable to CABG, HCR and multivessel PCI, the quantitative endpoint of residual myocardial ischaemia at 12 months, which is predictive in a gradient manner of cardiac death and adverse cardiac events, was similar with all three guideline-accepted revascularisation strategies. MVCAD PCI, using contemporary best-in-class drug-eluting stents, was associated with a shorter hospital stay, less inpatient rehabilitation and shorter periods of sick leave than CABG or HCR.93 While extended follow-up will determine longer-term outcomes from their study, a larger-scale multicentre trial powered for clinical endpoints would be warranted.
Conclusion
In the five decades since it has been introduced, CABG has been subject to continuous improvements and changes. The way in which the procedure is now performed has been transformed by technological advances that have propelled forward multiple CABG techniques. In the current era, CABG has become less invasive and emphasis has been given to more patient-friendly approaches and more durable results. MIDCAB was first described by Calafiore et al. and since then, many studies have highlighted the beauty of minimally invasive coronary procedures, accentuating it as an attractive alternative to conventional CABG as it bypasses the need for sternotomy.4 These less invasive methods are linked to reduced postoperative hospital stay, higher safety and higher efficacy and a better quality of life. When not performed as the primary operation, multiple studies have shown that MIDCAB can be performed in cases of reoperation.94–99 As opposed to CABG reoperations, MIDCAB has proven to be more effective and not linked to increased mortality and morbidity.100,101 MIDCAB is also a viable alternative to CABG and can also replace PCI in patients for whom PCI is either risky or impossible.
A major obstacle physicians face when initiating MIDCAB is finding criteria for optimal patient selection. A further issue is that MIDCAB is technically demanding and accounts for longer learning curves making it prone to anastomotic failure if surgeons are not experienced. MIDCAB is also more costly in comparison to bare metal stenting. One of the newer developments in cardiac surgery is robotic-assisted MIDCAB and TECAB, aimed at yielding effective and lasting coronary anastomoses as well as faster recovery and less bodily trauma. It can, nonetheless, also affect cardiac and pulmonary function and cause prolonged mechanical ventilation. However, this seemed to be linked to certain pre-operative patient-related risk factors.









