The optimal management strategy for percutaneous coronary intervention (PCI) of bifurcation lesions has received significant interest in the scientific literature and remains a matter of debate among the interventional cardiology community. A bifurcation lesion is defined by the European Bifurcation Club as a “coronary artery narrowing occurring adjacent to, and/or involving, the origin of a significant side branch”.1,2 Bifurcation lesions are encountered in approximately 21% of PCI procedures, and they are recognised to confer an increased risk of major adverse cardiac events (MACE).3,4 There is considerable variation in bifurcation lesion anatomy. The Medina classification has been developed in an attempt to standardise the terminology when describing these lesions.
In this article, we focus specifically on the management of the Medina 0,0,1 lesion (‘Medina 001’). This is an uncommon lesion that is encountered in <5% of all bifurcations.5 The aim of this paper is to discuss important technical considerations with regard to the treatment of Medina 001 lesions and to describe the current published data supporting the various proposed interventional treatment strategies. In this article, Medina 001 lesions involving the left main stem (LMS) are excluded from the discussion. The rationale for this is because LMS PCI carries its own particular considerations, which are outside the scope of this review.
Laws of Bifurcation Anatomy
Like many other natural phenomena, the coronary arterial tree exhibits ‘fractal geometry’ (i.e. self similarity and scale invariance) as it branches into smaller vessels. Murray’s law states that: “When a parent blood vessel branches into daughter vessels, the cube of the radius of the parent vessel is equal to the sum of the cubes of the radii of daughter blood vessels.”6 This means that if a branch of radius r splits into two branches with radii r1 and r2, then r3 = r13 + r23. Murray’s law has been used to estimate vessel sizing in coronary bifurcations. Finet et al. have also proposed a simple formula for estimating the diameter of the mother vessel (Dm) and daughter vessels (Dd1, Dd2) based on a simple fractal ratio, and this rule has also been used to estimate vessel sizes:
Dm = 0.678 × (Dd1 + Dd2)
where Dm is the diameter of the mother vessel and Dd1 and Dd2 are the diameters of the two branching vessels.7
Bifurcation Classifications and Medina 001
The Medina classification describes a bifurcation lesion with three numbers that aim to define the pattern of the atherosclerotic disease based on angiographic appearance.8 Each number is designated either 1 or 0 to indicate the presence or absence of a significant (>50%) stenosis. The first number refers to the proximal main branch (MBprox), the second refers to the distal main branch (MBdistal) and the third refers to the side branch (SB) ostium.8 Lesions that involve both the main branch (MB) and SB are often defined as ‘true bifurcation’ lesions, whereas lesions involving only one of the MB or SB are referred to as ‘non-true bifurcation’ lesions.8 The ‘Medina 001’ indicates isolated ostial SB disease with no disease of the MB. This type of lesion has also been described in the literature as an ‘ostial SB lesion’. There are four other prominent bifurcation classifications described in the literature.9–12 These all share similar principles, but most of these classifications have failed to make the transition into daily clinical practice.
Defining the Medina 001 Lesion
The initial classification of a bifurcation is often based entirely on angiographic appearance. However, with the use of adjuvant imaging modalities the pattern of bifurcation may be redefined. This is particularly relevant in Medina 001 lesions because the definition of the disease pattern may have important implications with regard to the optimal management approach.
Adjuvant modalities that may provide extra information to classify the pattern of disease include quantitative coronary angiography (QCA), intravascular imaging with optical coherence tomography (OCT) or intravascular ultrasound (IVUS) and physiological assessment with fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR).
Dedicated QCA software can provide more information than simple angiographic analysis. This includes the three reference diameters (MBprox, MBdistal and SB), lesion length, percentage stenosis and bifurcation angles. However, QCA was initially developed and validated in single, straight coronary segments, and concern remains regarding the accuracy of dedicated bifurcation lesion software and potential inter- and intralaboratory variability. Improvements to available dedicated bifurcation QCA software and novel 3D QCA bifurcation software may help surmount some of these issues and increase the use of these modalities in clinical practice.
Intravascular imaging with IVUS or OCT can provide a wealth of information on bifurcation anatomy: it has the ability to accurately measure the true lumen size, vessel diameters, plaque burden and morphology, stent landing zones, stenosis severity and lesion length.13 However, with specific regard to Medina 001 lesions, it should be noted that both OCT and IVUS parameters can have a low positive predictive value for determining the functional significance of SB ostial lesions.14
Physiological assessment of bifurcation anatomy with FFR or iFR can also provide additional ‘functional information’ regarding a lesion that may help guide management. FFR is often considered the gold standard for identifying myocardial ischaemia in the cardiac catheterisation laboratory, and it has been shown to be safe to defer PCI in FFR-negative lesions.15 Götberg et al. demonstrated that among patients undergoing functional determination of an indeterminate coronary stenosis for either stable or unstable coronary disease, iFR was non-inferior to FFR in guiding PCI.16 This is particularly relevant for ostial SB lesions, where documentation of negative FFR may obviate the need for PCI. One must be aware of creating a false dichotomy with regard to FFR or iFR positivity, because a positive result does not indicate that PCI must be performed. It is important to note that the benefit observed in the FAME studies was primarily in patients with an FFR value of <0.65.17 As such, it is important to adopt a nuanced approach and recognise that an FFR value of 0.79 or an iFR value of 0.88 is not an absolute indication for PCI. Instead, this may serve as a starting point to consider a patient’s symptom burden, their medication regimen and to incorporate all this information in order to determine the best way to move forward and manage their disease.
Management of Medina 001 Bifurcations
When encountering a Medina 001 lesion in clinical practice, there are several important factors to consider. Prior to performing invasive intervention, the physician should consider the lesion morphology (with angiographic/intravascular imaging guidance), the presence of ischaemia in the associated territory (non-invasive/invasive documentation) and the clinical scenario (acute coronary syndrome, chronic coronary syndrome, anginal symptom burden). The interventionist must also remain cognisant of the complex interplay between angiographic appearances, symptom burden and documented myocardial ischaemia. Given the risk of MB compromise associated with Medina 001 bifurcation lesions, it is important that the PCI approach is reserved for patients in whom it is truly indicated.
With this in mind, there are some pivotal trials that should be considered when discussing the management of Medina 001 lesions. In 2007, the COURAGE trial found no benefit with revascularisation over optimal medical therapy in stable coronary artery disease (CAD).18 The limitations of that study included the randomisation of patients after angiography and the use of bare metal stents. There was also a suggestion that patients with more severe disease would benefit from revascularisation. Subsequently, the ISCHEMIA trial highlighted that, among patients with stable ischaemic heart disease and moderate to severe ischaemia on non-invasive stress testing, a routine invasive strategy failed to reduce major adverse cardiac events compared with optimal medical therapy.19 There was evidence for symptomatic benefit, but it may have been confounded the lack of a blinded sham procedure in the control group. This is important because the ORBITA trial had previously demonstrated that in patients with medically treated angina and a single-vessel, severe coronary stenosis, PCI did not increase exercise time by more than the effect of a sham control procedure.20 Together, these trials remind us of the limitations of PCI with regard to the management of stable CAD. This is particularly relevant when discussing the management of Medina 001 lesions, because the physician must always consider the associated risk of causing MB compromise.
When considering this risk–benefit ratio, it is important to remember that Medina 001 lesions will tend to be of lesser prognostic importance that the MB and will supply a relatively small myocardial territory. For example, in a prospective study of 65 patients with left anterior descending (LAD) artery bifurcation lesions, diagonal branch occlusion resulted in lower rates of anginal chest pain (40% versus 100%; p=0.001), ST segment change (37% versus 92%; p=0.001) and arrhythmia compared with occlusion of the LAD.21
Another multicentre registry of 482 patients undergoing coronary CT angiography (CTA) and FFR measurement reported that only one of every five non-left main SBs (n=2,448) supplied a percentage fractional myocardial mass (%FMM) >10% (97% versus 21%; p<0.001).22 Compared with the MB, the SB supplied a smaller myocardial mass and demonstrated less physiological severity despite similar stenosis severity. That study also suggested that an SB supplying a myocardial mass of %FMM ≥10% could be identified by vessel length ≥73 mm (C-statistic=0.85; p<0.001).22 This is a topic of continued debate, but these studies may offer some explanation as to why more aggressive treatment of the SB has failed to show clinical benefit in many coronary bifurcation trials.21,22
Hachamovitch et al. reported that revascularisation compared with optimal medical therapy only had greater survival benefit (absolute and relative) in patients with moderate to large amounts of inducible ischaemia (>10% ischaemia).23 Koo et al. also proposed the SNuH scoring system to estimate the mass of the myocardium at risk when intervening on diagonal SBs.21 The SNuH scoring system takes three factors into account in an attempt to determine the clinical significance of a diagonal branch: the size of the vessel (vessel diameter ≥2.5 mm=1 point), the number of diagonal branches (number ≤2=1 point) and whether it is the highest branch (no branch below target branch=1 point). However, this scoring system had low positive predictive value, and this highlights the limitations of angiographic assessment in determining the clinical significance of a diagonal SB.21
Taking these data into account, optimal medical therapy may be a reasonable initial strategy for Medina 001 lesions identified in stable patients, with PCI reserved for patients with refractory anginal symptoms. An observational study of Medina 001 lesions by Brueck et al. lends credence to this approach.24 These authors compared 233 medically managed patients with 69 who were treated with angioplasty for ostial diagonal disease ≥2 mm. PCI resulted in increased rates of rehospitalisation (55% versus 22%; p<0.001) and revascularisation (23% versus 8%; p<0.001) compared with conservative therapy.24 However, these data are non-randomised and so should be interpreted with caution.
Technical Considerations in Treating Medina 001 Lesions
The overriding concern when performing PCI for a Medina 001 lesion is the possibility of compromise or injury to the main vessel. This may occur either immediately at the time of intervention or present at a later stage. As the main vessel (by definition) subtends a larger amount of myocardium, a periprocedural myocardial infarction involving the main vessel when the initial aim was to intervene on a smaller SB vessel is a complication that should be avoided at all costs.
It should be recognised that ostial lesions can be fibrotic and calcific, and this may confer a higher risk of stent underexpansion and stent restenosis. Changes in flow and shear stress at the bifurcation likely contribute to this lesion morphology. In continuous segments, flow within the vessel is linear and applies force on the vessel wall, described as wall shear stress (WSS).25,26 Reduced WSS has been associated with the development of atherosclerosis.27 Reduced WSS is commonly seen in coronary bifurcations where flow becomes turbulent, slow and occasionally reversed.25,28 Prior to the level of flow separation, blood flow is brisk and linear. However, in segments opposite the carina in the MB and SB, flow becomes turbulent and oscillatory.25 Histopathological and IVUS analyses have demonstrated that atheroma is frequently located at bifurcations and tends to form in segments with reduced WSS.28,29 Most often, the carina itself is free of atheroma due to these flow dynamics. The pattern of in-stent restenosis can be similarly affected by the same flow mechanics after bifurcation PCI.30 The different flows and shear stresses involved in bifurcation lesions have important implications that may contribute to the increased risk of MACE associated with bifurcation stenting.31,32
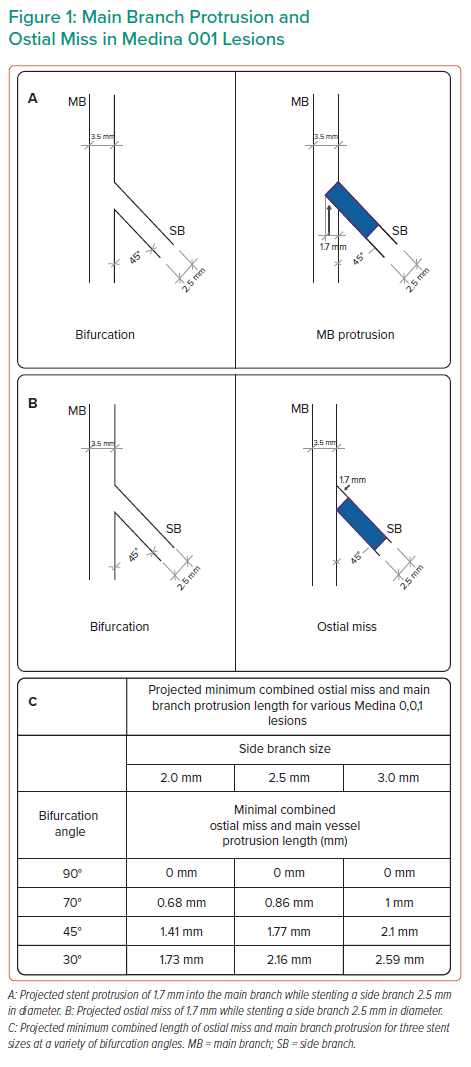
The intertwined concepts of ‘ostial miss’ and ‘MB protrusion’ deserve special mention with regard to Medina 001 lesions. ‘Ostial miss’ refers to the stent being placed too distally and missing the ostium of the SB. ‘MB protrusion’ refers to the stent being placed too proximally and protruding into the MB. This is demonstrated in Figure 1. Operators often speak of landing the stent ‘right at the ostium’ or ‘nailing the ostium’, and various techniques have been described to facilitate achieving this goal. However, as can be seen in Figure 1, with the exception of 90° bifurcation angles, it is not possible from a geometric standpoint to place a stent right at the ostium. For all other angles, there will be at least some degree of ostial miss and/or MB protrusion, however minimal. In Figure 1, we have calculated the projected minimal combined length of ostial miss and MB protrusion for three stent sizes (2.0, 2.5 and 3.0 mm) implanted in a Medina 001 lesion for a variety of bifurcation angles. This was calculated based on trigonometric analysis of a simplified 2D geometric model of a stent in a Medina 001 lesion, as seen in Figure 1. This combined minimum value remains constant. Reducing the ostial miss length will increase the MB protrusion by the same amount, and vice versa. Interventionists should also note that for bifurcation angles of ≤60°, a minimum combined ostial miss/main vessel protrusion value of >1 mm is inevitable. Both ostial miss and main vessel protrusion may increase the subsequent risk of restenosis in both the main vessel and SB, and therefore should be avoided.
Interventional Management
An interventional strategy for the management of Medina 0001 lesions can be divided into techniques that include stenting and those that do not. Lesion preparation is crucial prior to stenting, particularly if the lesions are fibrotic or calcified. If a non-stenting approach is used, the interventionist must be careful not to dissect the vessel and be ready for a bail-out stenting strategy. It is important that this is planned for prior to the PCI and that operators are cognisant that, in some studies, ‘bail-out’ stenting has been associated with an increased risk of mid-term MACE after PCI.33 Operators should always have a Plan B in mind and prepare accordingly. Adjuvant interventional techniques to adequately prepare a Medina 001 lesion may include cutting balloons, scoring balloons, rotational atherectomy and/or intracoronary lithotripsy, as appropriate.
Stent underexpansion due to inadequate lesion preparation is a common cause for stent failure.34 These lesions may be technically difficult, and this pattern of calcified and fibrotic disease is often seen in older patients with a greater number of comorbidities, including diabetes, chronic kidney disease and hypertension.35 Deployment of a stent in an unprepared or inadequately prepared lesion may result in stent underexpansion. This should be avoided if possible, because residual stenosis following stent insertion is a major determinant of restenosis after PCI.36
Non-stenting Techniques
Plain Old Balloon Angioplasty
Plain old balloon angioplasty (POBA) was one of the earliest treatment strategies used to treat coronary bifurcation lesions. However, POBA procedures were associated with a low rate of procedural success and frequent complications, such as recoil, dissection and restenosis secondary to intimal disruption.37,38 There has been no dedicated randomised control study on POBA in Medina 001 lesions.
Drug-eluting Balloon Treatment
Drug-eluting balloons (DEB) are semicompliant angioplasty balloons coated with an antiproliferative drug (usually paclitaxel) that is rapidly released upon contact with the vessel wall.39 Stenoses are ideally pretreated with standard balloon angioplasty, a non-compliant balloon and/or scoring or cutting balloons prior to the use of a DEB.40 Once an adequate initial balloon angioplasty result is obtained, the DEB can be inflated for up to 90 s to permit sufficient drug transfer.
DEBs have emerged as a potential alternative to drug-eluting stents. One of the benefits associated with the use of a DEB in the management of bifurcation lesions includes homogeneous administration of the drug to the coronary wall, which may negate some of the restenosis seen in comparison with POBA. There may also be less disruption of the carinal anatomy compared with stenting, and the required duration of dual antiplatelet therapy can be reduced. Difficulties encountered with DEBs include issues with antiproliferative drug release, elastic recoil of lesions and coronary dissection.
A 3-year multicentre observational registry by Vaquerizo et al. recruited 49 patients with Medina 001 lesions and associated myocardial ischaemia.41 The lesions were prepared carefully using gradually increased inflation pressures in order to reduce the risk of dissection. Once an optimal dilatation result was obtained (defined as a residual stenosis <50%), a Dior paclitaxel DEB (5 mm longer than the predilating balloon) was inflated for >45 seconds. Subsequent bail-out stent implantation was only required in 14% of patients. At 1 year, the rate of target lesion revascularisation was 14%.41 That study showed that DEBs are a safe and technically feasible therapeutic option for the treatment of Medina 001 lesions. However, as an observational registry, it is hypothesis generating at best, and further dedicated randomised control trials are required to compare DEB to drug-eluting stents in the management of Medina 001 lesions.42–44
From a theoretical standpoint, the DEB has several advantages for Medina 001 lesions. The operator can ensure that the ostium is treated without requiring the implantation of a permanent stent. This removes the previously discussed issue of ostial miss/main vessel protrusion from the equation. At present, DEBs represent a viable option for the treatment of Medina 001 lesions, particularly when SB stenting is not desired. However, randomised data are required to make definitive statements in this regard.
Rotational Atherectomy With or Without Plain Old Balloon Angioplasty
Rotational atherectomy (RA) of the MB has been described in the literature for a wide variety of bifurcation lesions. RA has been postulated to be beneficial in the removal of calcified plaque in front of the SB.45,46 There are limited data regarding the use of RA for ostial SB or Medina 001 lesions. A 26-month prospective observational study reported on the clinical and angiographic outcomes of 105 patients with ostial lesions who underwent RA of SB lesions.47 Supplementary POBA was used if a residual stenosis ≥30% persisted despite appropriate burr sizing or if an angiographic complication developed. Following RA, the mean ± SD percentage diameter stenosis was reduced from 73 ± 13% to 41 ± 14% (p<0.001); adjunct balloon angioplasty was used in 89 procedures (85%), resulting in a 23 ± 14% final diameter stenosis (p<0.001).
Procedural success was achieved in 97% of patients.47 Several complications were observed, including minor coronary dissections in 18 patients, coronary spasm in three patients and post-procedural coronary thrombus in three patients. During the 5.4 ± 3.6 months of follow-up, 34% of patients developed recurrent symptoms. Angiographic restenosis was seen in 32% of patients eligible for the 6-month follow-up. Four patients died during follow-up, including three deaths from cardiac causes.47 Although RA has been demonstrated to be an effective tool in other lesion subsets, there remain no randomised data in Medina 001 lesions. Therefore, given the higher complication rates associated with this technique, caution should be advised in the use of RA for Medina 001 lesions at present.
Stenting Strategies
If the decision is made to use a stent, there are several techniques that have been proposed as being particularly suited to Medina 001 lesions. In this section we will describe some of these techniques and, where available, the evidence behind them.
Flush Ostial Technique
The simplest technique is the flush ostial technique. This is colloquially referred to as ‘nailing the ostium’ and involves simply attempting to place the stent exactly at the ostium of the SB. Unfortunately, as discussed above, unless the angle of the bifurcation lesion is at 90° there will inevitably be some degree of ostial miss or MB stent protrusion with this technique, which increases the risk of restenosis of the main vessel and SB.
Stent Draw-back Technique
In the early 2000s, Schwartz and Morsi described the stent draw-back technique.48 In this technique, a stent is located in the SB with a balloon in the main vessel inflated to relatively low pressures. The SB stent is pulled back against the inflated balloon until a dent is seen. The stent is then deployed, and the balloon removed thereafter.49 A major disadvantage of this technique is there will inevitably be some degree of injury caused to the main vessel intima due to balloon inflation.
An observational series of 100 patients (pull-back = 55, conventional technique = 45) demonstrated a procedural success of 100%.49 The data also suggested that the stent draw-back method was most suited to wide angle bifurcations. As mentioned above, this approach does not overcome the geometrical limitations of minimal combined ostial miss/main vessel protrusion and will only serve to ensure that the combined ostial miss/main vessel protrusion value is as close as possible to the minimum value.
Szabo Technique
The Szabo technique consists of ‘pushing’ the SB stent over the target vessel wire while a second anchor wire is present in the proximal strut of the stent. The stent is inflated to a low pressure (<4 atm) to allow advancement of the second guide wire. Alternatively, the distal strut may be manually lifted. Following this, the stent is manually recrimped onto the balloon. The stent is delivered over both guide wires and deployed (at 8 atm). The anchor wire is extracted with the stent deployed at high pressure.50 The anchor wire advancing through the proximal stent strut helps prevent excessive protrusion of the stent past the ostium while promoting accurate ostial stenting. It should be emphasised that, again, if the angle is <90°, although the Szabo technique may reduce MB protrusion/ostial miss, it will not eliminate it entirely.
The Szabo technique has been demonstrated to be accurate in positioning of the stent due to the protective measures in halting distal advancement. One study achieved almost near perfect successful stent implantation position with IVUS in 40 of 41 patients treated with the Szabo method.51 However, most of these were not Medina 001 lesions. Another retrospective study of a registry compared 78 Medina 010/001 lesions or aorto-ostial lesions treated with the Szabo technique to 179 lesions treated by conventional means.52 The authors reported that compared with conventional treatment, the Szabo technique reduced the incidence of stent malposition (6.4% versus 41.0%; p=0.000001) and reduced incomplete scaffolding of the plaque (0.0% versus 7.7%; p=not applicable).52 However, this was based on angiographic analysis rather than intravascular imaging.
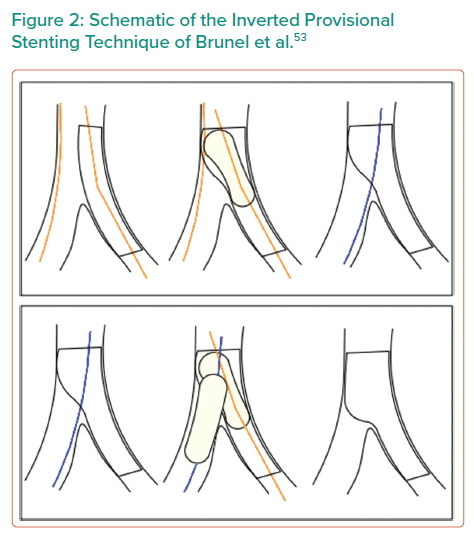
Inverted Provisional Stenting/Crossover Technique
The inverted provisional strategy involves placing a stent from the proximal main vessel into the SB. It can be completed as a true provisional technique with only an MB proximal optimisation technique or it can be completed as a provisional technique with kissing balloon inflation and final proximal optimisation, shown in Figure 2.53 The advantage of this technique is that the ostium is completely covered, although this is achieved at the expense of stenting back into the non-significantly diseased main vessel. In addition, it is most suitable for situations where there is not a large size mismatch between the two vessels. If there is a significant difference in size between the MB and SB, stent selection requires particular attention to the stent expansion capabilities and limitations. If compromise to the main vessel occurs with this technique, a bail-out two-stent strategy is relatively straightforward via either T and small protrusion (TAP) stenting or a culotte strategy.
Brunel et al. reported their experience of inverted provisional T stenting in a registry of 40 patients.53 With this technique, they implanted the stent from the proximal MB through the SB, reopened the struts through the distal MB and finished with a systematic final kissing balloon inflation. Brunel et al. reported complete coverage of the ostial SB in 100% of cases with this technique based on angiographic appearance.53 This allowed the authors to achieve a successful procedure with implantation of a single stent in 92.5% of cases. There were no MACE at 30 days and an acceptable re-intervention rate of 5% at a median follow-up of 22 months.53
Another interesting variation on the inverted provisional technique uses the dedicated Tryton SB stent. Grundeken et al. reported this technique using the Tryton Side Branch Stent (Tryton Medical) without a stent in the main vessel.54 Although the authors reported 100% procedural success and only one late clinical adverse event in a small series of 12 patients, there is limited evidence beyond this small series to support this strategy.
Crush Technique Without Main Vessel Stenting
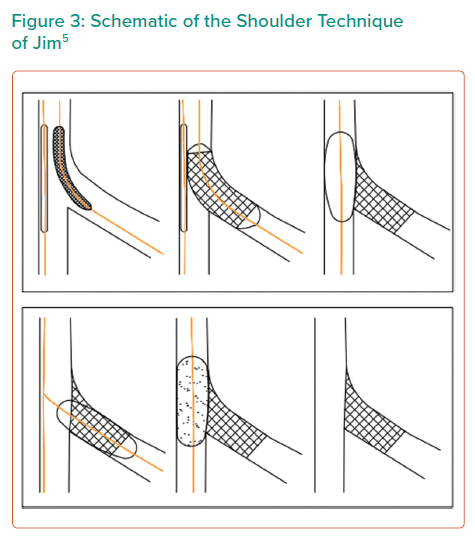
In the crush technique without main vessel stenting, a stent is deployed in the SB with minimal protrusion into the MB. The wire in the SB stent is then removed and a balloon in the MB is used to crush the SB stent flush against the ostium. The SB stent is then rewired and the procedure completed with kissing balloon inflation. This ensures complete SB coverage. Limitations of this strategy include that it requires ballooning in the main vessel.
A similar technique was developed in 2014 by Jim et al. (Figure 3).5,55 In this technique, the SB stent is again crushed, but no kissing balloon inflation is performed. Instead, the SB stent is rewired and the lumen of the SB ostium is dilated with high-pressure balloon inflation. Subsequently, a DEB is inflated in the MB. The name of the technique derives from the resultant SB stent appearing like ‘the shoulder of a sleeve’. There are no studies looking at the long-term outcome of the crush technique in Medina 001 lesions.
Modified Flower Petal Technique
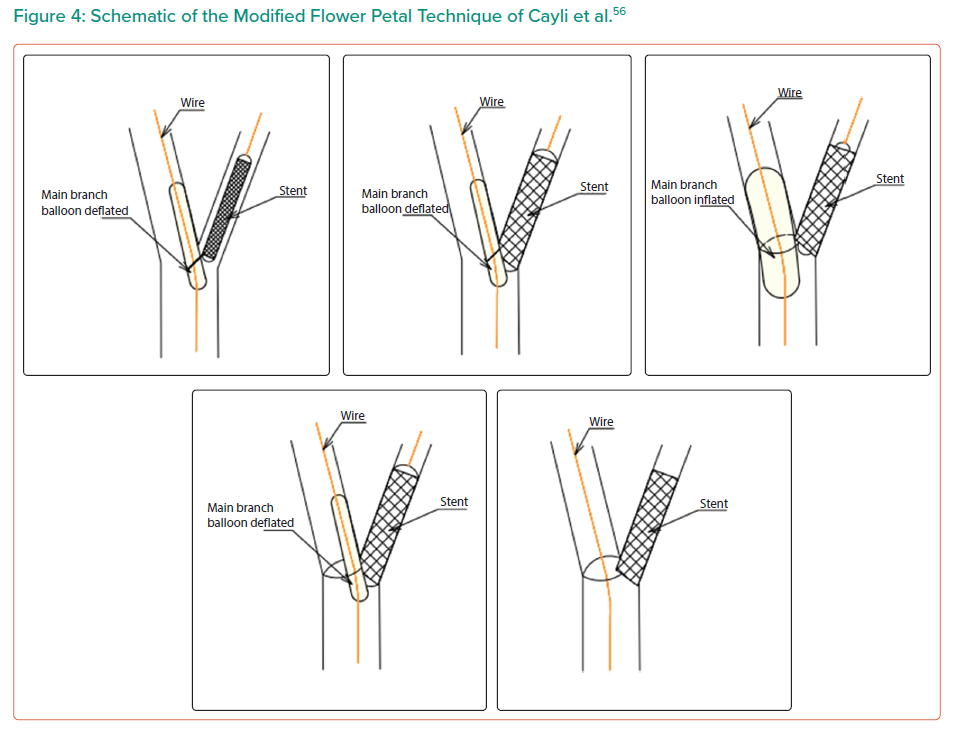
The modified flower petal technique was proposed by Cayli et al. for Medina 010 and 001 lesions.56,57 A registry of 64 patients was analysed, which included both Medina 010 (n=34) and Medina 001 (n=30) lesions. This technique is a modification of flower petal stenting, first described by Kinoshita et al.58
In this modified technique, both branches are initially wired (main vessel and SB). The first wire enters the SB, with the second wire entering the main vessel to act as an anchor (Figure 4).56 Once both branches are wired, a stent balloon system must be prepared outside the guiding catheter. To do this, the plastic stent cover must be pulled back so that the final proximal stent strut is exposed. The stent delivery balloon is inflated to low pressure (5–6 atm) and then deflated. Then, the proximal end of the anchor wire is passed through the final proximal stent strut and another balloon is loaded on the anchor wire as an anchor balloon. The proximal markers of both the stent and balloon are aligned, and the final proximal strut of the stent can then be recrimped by hand. The stent balloon system is now complete.
This stent–balloon system can then be passed through the guiding catheter to the target lesion until the anchor balloon halts the continuation of the stent. The concept is that the anchor balloon can avert excessive stent advancement into the target branch. In the technique described by Cayli et al. the stent balloon is firstly inflated and deflated.56,57 The anchor balloon is then inflated and deflated and the stent balloon is the inflated once more. After this, the protruding final proximal strut is in contact with the opposite side of the adjacent vessel wall, the ‘flower petal’ from which the technique derives its name.
The proposed advantage of this technique is the total coverage of the ostial bifurcation lesion with stent struts without encroachment into the main vessel, and a lower metallic burden at the carina segment. At the 9-month follow-up, data were only available for 59 patients.56 There were no cardiac deaths, MIs or stent thrombosis events. Only one patient (1.7%) had binary restenosis on quantitative coronary angiography. Difficulties encountered with this technique included twisting of wires, seen in 10 patients (15.6%).
Conclusion
The Medina 001 lesion is a rarely encountered lesion that presents unique challenges with regard to ostial miss, main vessel protrusion and the potential for main vessel injury and compromise. There is a paucity of high-quality, randomised control data to guide the interventional management of Medina 001 lesions.
Most Medina 001 lesions will not supply more than 10% of the myocardium, and so it is important that intervention to these lesions does not compromise the MB. Intravascular imaging and physiological assessment may provide extra information to help guide management and accurately classify the anatomy and functional significance of the bifurcations.
Based on current evidence, medical management should likely retain its position of primacy at present, with PCI reserved for unstable Medina 001 lesions presenting as an acute coronary syndrome or lesions causing refractory angina after a period of medical optimisation.
If stenting must be performed, operators must accept that, with the exception of 90° bifurcation angles, some degree of ostial miss or MB stent protrusion is inevitable, regardless of the strategy used, which may result in poor outcomes to either the main vessel, SB or both. With this in mind, the authors feel the most appropriate stenting strategy is the inverted provisional/crossover technique, which ensures complete ostial coverage. This technique is also relatively safe if compromise to the main vessel occurs, because placement of a second stent is reasonably straightforward.
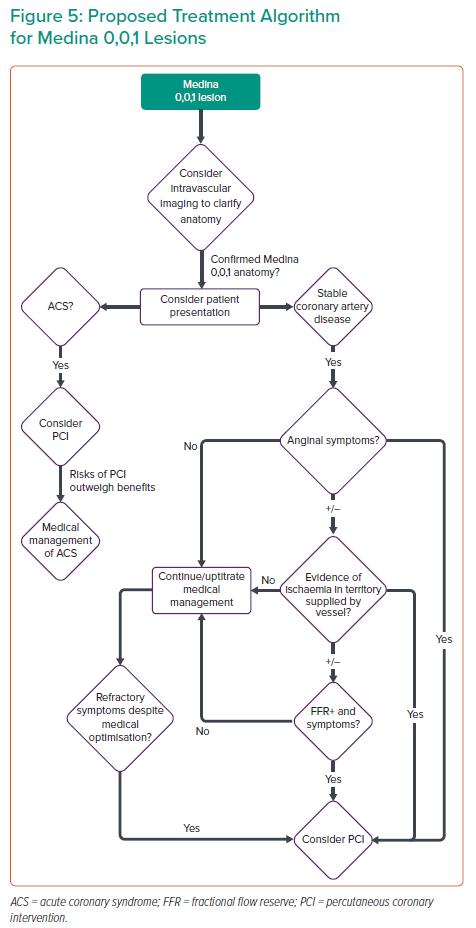
DEB treatment, with or without cutting balloon lesion preparation, has some theoretical advantages over stenting for Medina 001 PCI. However, randomised control data to support this are lacking at present, and this is an area for future research. In Figure 5, we present a proposed management algorithm for Medina 001 lesions.