Coronary bifurcations are encountered in about 15–20 % of percutaneous coronary interventions (PCIs). They are considered technically challenging and associated with worse clinical outcomes than non-bifurcation lesions. However, in recent years significant developments in the understanding as well as in the treatment of coronary bifurcation lesions have occurred.1 First, the development of drug-eluting stents (DES) has considerably decreased the rates of restenosis and repeat revascularisation. Second, the acceptance of a suboptimal result in the side branch (SB) appeared since it was proved that many residual stenoses at the SB might not be physiologically significant. And, finally, numerous randomised trials focused specifically on bifurcations have been published. As a result of these changes, the provisional approach of one stent implantation only in main vessel–main branch (MV–MB) is now considered the default approach in most bifurcation lesions.2
Also, the use of dedicated bifurcation stents might facilitate the procedure, even in complex and challenging anatomies. Dedicated bifurcation stents can be broadly divided into four categories: dedicated MV devices (e.g. Axxess, BioSensors BV, Hillegom, the Netherlands), dedicated MV with SB access port (e.g. Xience SBA, Abbott Laboratories, Abbott Park, Illinois, US; Nile Croco, Minvasys, Gennevilliers, France), dedicated SB devices (Sideguard, Cappella Medical Devices Ltd, Galway, Ireland; Tryton, Tryton Medical, Durham, North Carolina, US) as well as dedicated MV plus SB devices (Medtronic Bifurcation Y-Stent, Medtronic Bifurcation Y-stent, Medtronic, Minneapolis, Minnesota, US). However, neither of these stents match the MV–MB size difference nor take into account vessel angulations.3 The BiOSS® stent (Balton, Warsaw, Poland) is completely different from the above systems.
Device Description
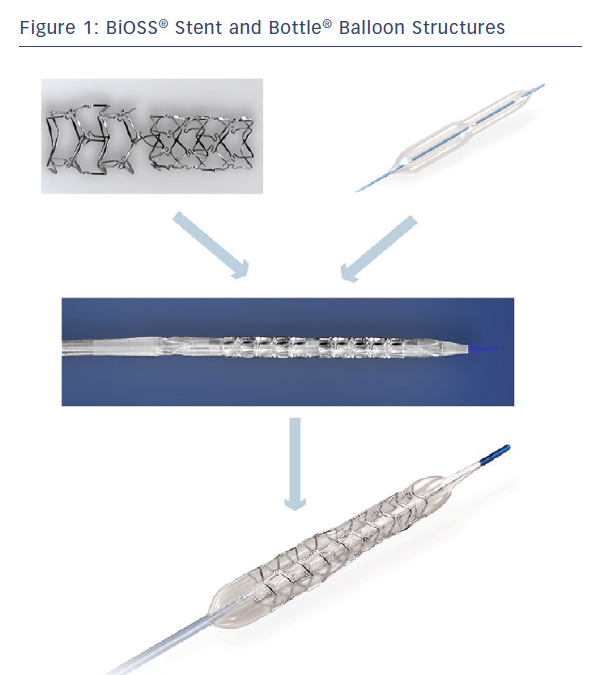
The BiOSS LIM® is a dedicated bifurcation balloon expandable stent made of 316 L stainless steel (strut thickness 120 µm) releasing sirolimus (1.4 µg/mm2) from the surface of a biodegradable coating comprised of a copolymer of lactic and glycolic acids (PGLA) (see Figure 1). The degradation of the polymer lasts approximately 8 weeks. The BiOSS LIM® stent consists of two main separate parts with different diameters: wider proximally, and distally smaller. The proximal part is always a bit shorter than the distal one (avergae 1 mm). The ratio of the proximal part to the distal one varies between 1.15 to 1.3, ensuring physiological compatibility and optimal flow conditions. There is a 2.0–2.4 mm middle zone with two connecting struts after the BiOSS® stent implantation. This zone ensures ‘self–positioning’ of a stent after balloon deflation, as well as the opening to SB. There are three lengths (15, 18 and 23 mm) of BiOSS® stents available on the market. The nominal foreshortening of the stent is less than 0.5 % and the stent strut/vessel area ratio varies between 15–18 %.4
The stent is crimped on a bottle-shaped balloon (Bottle®, Balton, Warsaw, PL) (see Figure 1). Bottle® balloons are available in a wide range of sizes and lengths allowing the left main (LM) treatment as well. The balloon nominal pressure is 10 atm, whereas the rated burst pressure is 18 atm. The balloon is semi-complaint with an increase in a diameter size of 0.25 mm at 12 atm, both proximally and distally.
The rapid exchange delivery system for the BiOSS LIM® stent is compatible with 0.014” guide wires and with 5 Fr (1.63 mm internal diameter) guiding catheters. The BiOSS LIM® stent is introduced over a single guide wire, which (contrary to other dedicated systems guided on two guide wires) eliminates the risk of wire wrap (twisting) or other complications with double guide wire-driven systems. Worth mentioning is the fact that the BiOSS LIM® stent was also implanted in bifurcations located in peripheral arteries, i.e. below the knee.5
BiOSS LIM® – The Mechanism of Action
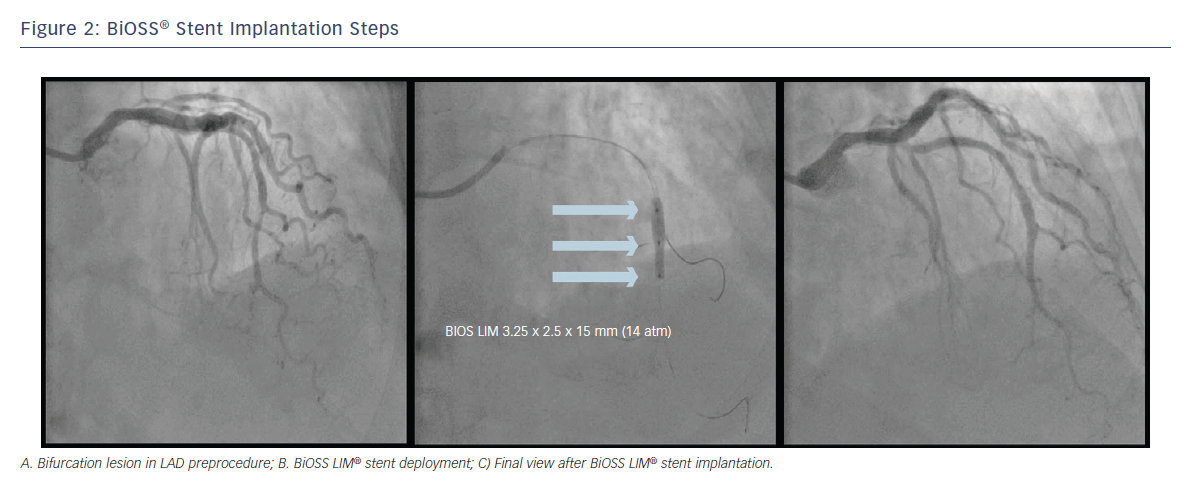
After wiring the MB and the SB, predilations can be performed according to the operator’s preferences. The BiOSS LIM® stent’s delivery balloon has three markers: distal and proximal indicating stent edges and one mid-marker showing the mid-zone. The mid-marker should be placed exactly at the tip of the carina. This ensures that after deployment the contralateral to SB wall is covered with struts to the same extent as the proximal MV part of the bifurcation (see Figure 2). This is achieved by the self-centring properties of the device (due to special shape of connecting struts) and the ‘closure’ configuration between the proximal and distal parts of the stent. The BiOSS LIM® design reduces the risk of carina shift as well as the SB ostium compromise.6 Additionally, the Bottle® balloon shape ensures the proximal optimisation technique (POT)-like effect immediately after BiOSS® implantation; however, post-dilation of its proximal part (real POT) seems to improve clinical results.7 Also, its ease-of-use allows the implantation of two BiOSS LIM® stents in a culottes fashion in distal LM stenosis.8
Since the beginning, the BiOSS LIM® stent construction provoked the question whether a 2.0–2.4 mm long middle zone of that stent was the weakest part predisposing to restenosis and intrastent thrombosis. An intravascular ultrasound (IVUS) study on the device in non-LM bifurcations disclosed a different mechanism of lumen enlargement in coronary bifurcation lesions treated with a provisional approach between classic DES and the BiOSS® stents. Although, the comparable luminal gain was observed, the BiOSS® stent was associated with less luminal compromise and plaque redistribution at the level of the SB in-flow in the bifurcation segment.6 Moreover, the analysis of restenosis patterns in POLBOS I, in the FIM BiOSS LIM® Registry, as well as in the BiOSS LIM® in LM registry denies those assumptions.7,9,10
BiOSS® Clinical Programme
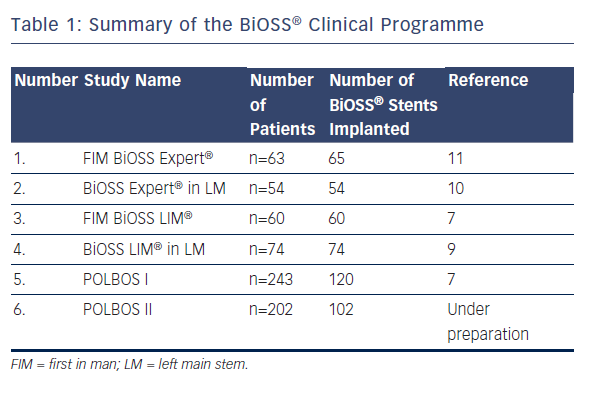
The BiOSS® (Bifurcation Optimization Stent System) Clinical Programme began in 2008. The first BiOSS® stent was bare metal, but shortly afterwards a paclitaxel-eluting version was introduced to the market – the BiOSS Expert® stent. A summary of previously gathered data is presented in Table 1. After acceptable results of the BiOSS Expert® stent in the all-comer population,11 as well as in distal LM stenosis,10 a way for improvement was to change the paclitaxel into the -olimus drug. The sirolimus was chosen and the BiOSS LIM® stent was developed.
BIOSS LIM® Registries
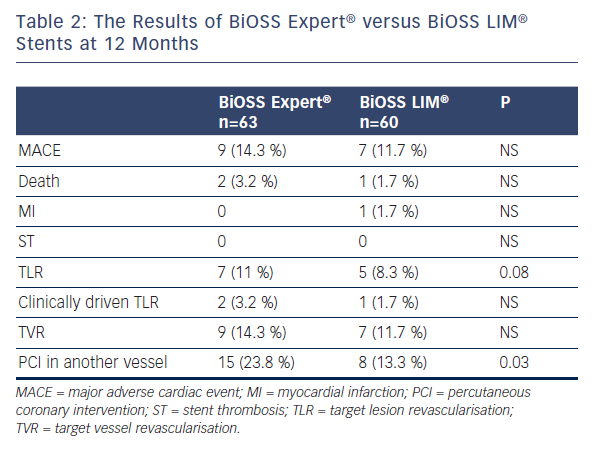
In the First-in-Man trial, 60 patients from three countries were enrolled (mean age 66.4±11 years, 28.3 % of female): 21.7 % of patients with non-ST-segment elevation acute-coronary syndromes (NSTE-ACS), 78.3 % with hypertension, 38.3 % with diabetes, 28.3 % had previous myocardial infarction (MI) and 46.7 % and 10 % underwent prior revascularisation: PCI and coronary artery bypass graft, respectively. At 12 months, the cumulative major adverse cardiac event (MACE) rate was 11.7 %. During follow-up (11±1 months) there was one non-cardiac death (1.7 %), one non-ST-elevated MI (1.7 %) due to restenosis and no case of stroke or in-stent thrombosis. Overall target lesion revascularisation (TLR) was 8.3 % (clinically driven TLR 1.7 %; angiographically driven 6.6 %). The SB was treated with an additional classic DES implantation in 23.3 % of cases.12 The comparison of BiOSS Expert® FIM and BiOSS LIM® FIM results are presented in Table 2.
In the second registry, 74 cases with distal LM were analysed. Seventy-three of 74 patients (aged 67±9 years, 23 % women, 20.3 % NSTE-ACS, SYNTAX score 22.4±4.4) were successfully treated with the BiOSS LIM® stent, with additional SB classic DES placement in 11 patients (14.9 %). Periprocedural MI occurred in one (1.4 %) patient. The 12-month MACE rate was 9.5 % without cardiac death or definite stent thrombosis. TLR and MI rates were 6.8 % (n=5) and 2.7 % (n=2), respectively. This report showed that implantation of the dedicated bifurcation BiOSS LIM® stent in distal LM stenosis at patients with moderate SYNTAX score was safe and effective. Also, these results suggested that the sirolimus-eluting BiOSS LIM® stent have better results than the paclitaxel-eluting BiOSS Expert® stent. Moreover, this stent might pose an interesting option in coronary bifurcation treatment, especially when there is a large difference in the diameter between the MV and the MB.9
In both cases our experience showed that this device is extremely user-friendly. The vast majority of implantations were possible using radial access (more than 90 %) and 6 F compatible equipment (including also LM cases). Moreover, such a stent ensured ideal immediate efficacy (100 % device success rate!) It is worth stressing the fact of easy rewiring of SB after BiOSS® Expert stent implantation. This device success rate is clearly superior to all reported in the literature data about procedural success rate of other dedicated coronary bifurcation stents (70–98 % for other types of devices). This high success rate is a result of the obvious lack of problems frequently occurring with other dedicated devices, like guide wires criss-crossing, improper device orientation and a rather big profile of the device.
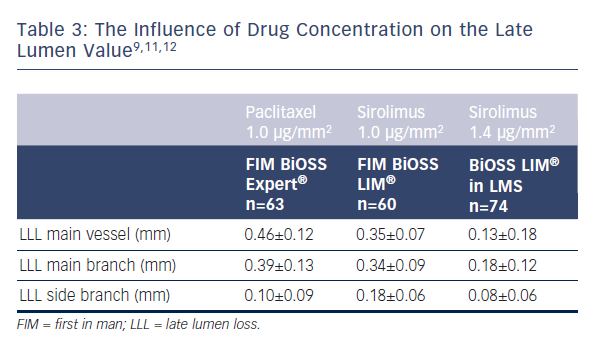
What must also be stressed during the BiOSS LIM® development different drug concentrations were used. As presented in Table 3 the use of higher concentration of sirolimus was associated with lower late lumen loss.
Randomised Clinical Trials
The POLBOS I study was the first randomised clinical trial.7 The aim of the POLBOS I trial was to compare bifurcation treatment with any classic DES to the dedicated bifurcation paclitaxel-eluting stent BiOSS Expert®.
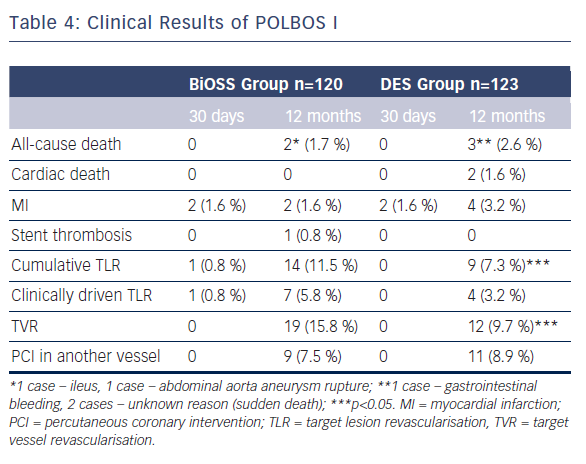
The second aim was to study the effect of final kissing balloon inflation (FKB) on clinical outcomes. Between October 2010 and January 2013 patients with stable coronary artery disease or non-ST-elevation acute coronary syndrome were assigned 1:1 to one of two treatment strategies: BiOSS Expert® stent versus classic DES implantation. Coronary angiography was performed at 12 months. The primary end-point was a composite of cardiac death, MI and TLR at 12 months. BiOSS Expert® was implanted in 120 patients (49.4 %) and DES in 123. The target vessel was LAD (52 % versus 70 %) followed by LM (22 % versus 15 %). In the DES Group, 38.2 % were paclitaxel-eluting stents. There were three stent implantation failures (two in DES; one in the BiOSS group). Side-branch treatment with DES was required in 10 % of cases in both groups. At 12 months, cumulative MACE incidence was similar in both groups: 13.3 % versus 12.2 % (p=0.7). The TLR rate was significantly higher in the BiOSS Group compared with DES: 11.5 % versus 7.3 % (p=0.02). In further analysis, when comparing the BiOSS Group to only the PES subgroup from DES, the rate of TLR in both groups was similar (11.5 % versus 10.6 %). Moreover, when comparing LM bifurcation versus non-LM bifurcation lesions BiOSS Expert® was significantly superior to DES in treatment of distal LM stenosis (TLR: 7.4 % versus 11.1 %, p=0.04). The rates of clinically driven TLR in our study were markedly lower in BiOSS as well as in DES Groups, 5.8 % and 3.2 % (NS), respectively. This is similar to the best results of DES in coronary bifurcation treatment (see Table 4).
Subgroup analysis regarding FKB versus no FKB revealed that in both groups (BiOSS Group and DES Group) FKB was related to a higher rate of SB stenting, longer time of fluoroscopy and treated lesions were more frequently localised in LM. However, in FKB subgroups there was a significantly lower rate of restenosis in the BiOSS Group
(8.1 % versus 13.2 %; p<0.05) as well as in DES Group (4.9 % versus 9.5 %; p<0.05). Interestingly, in the DES Group the rate of restenosis in the FKB+POT subgroup was even lower (1/42, 2.4 %). It strongly suggests the process of the BiOSS® stent implantation was optimised, despite its design.
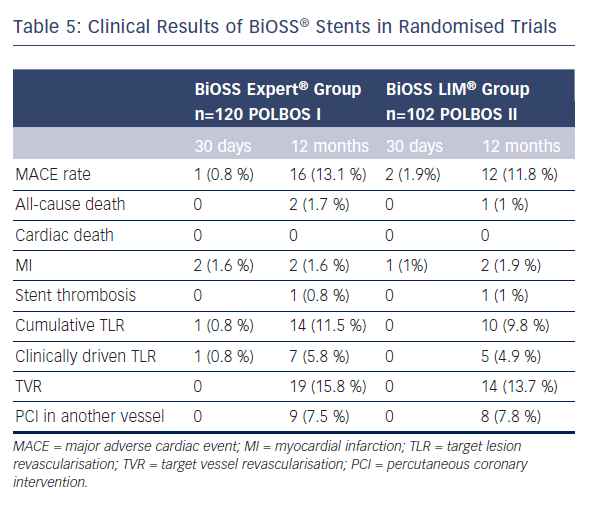
The POLBOS I trial established an important benchmark for future studies with new generations of BiOSS® stents eluting -olimus drugs and utilising newer stent materials. Therefore, the POLBOS II was the continuation of the concept of POLBOS I, where BiOSS LIM® was compared with regular DES. The interim analysis of POLBOS II and the final results of POLBOS I regarding BiOSS stents are presented in Table 5. Worth stressing is the fact that interim analysis of POLBOS II also suggests, similar to the POLBOS I study, that a more aggressive protocol (FKB and POT) during BiOSS® stent implantation yielded better angiographic and clinical outcomes.
The Future of BiOSS® Programme
Results of stainless steel thick-strut stent BiOSS® LIM look interesting. However, in real-life clinical scenarios this version seems not to be superior to the paclitaxel-eluting BiOSS® Expert stent. It is very likely that only the thin-strut BiOSS® -olimus eluting version (with higher, than the original, sirolimus concentration – 1.4 μg/mm2 instead of 1.0 μg/mm2) has the chance to provide better results compared with the new generation of regular -olimus eluting stents. Therefore, a cobalt-chromium (L605 alloy) BiOSS® stent is under development with strut thickness of 70 µm and the drug concentration of 1.4 µg/mm2 (polymer with drug layer thickness of 5 µm). And the international, multicentre, randomised trial POLBOS 3 with thin-strut BiOSS®
CrCo is being prepared, where it will be compared with the Xience® and Resolute® stents.
Conclusions
Our experience with BiOSS® stent let us conclude that this device is easy to use and compatible with modern low-profile stents. The elimination of carina displacement (as a main mechanism of SB compromise) by BiOSS® stent keeps the SB patent and do not require further treatment. The wide range of available sizes allows the majority of bifurcation patterns to be successfully treated.