The translation to clinical practice of agents or techniques that protect against the effect of ischaemia–reperfusion (IR) injury remains one of the most challenging areas of research in the field of cardiovascular medicine.1–4 This is particularly the case when IR injury follows revascularisation for acute MI (AMI). The publication of the combined Effect of Remote Ischaemic Conditioning on Clinical Outcomes in ST Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention (CONDI2/ERIC-PPCI) trial, which showed that remote ischaemic conditioning (RIC) did not reduce infarct size or improve cardiovascular outcomes, was a blow to the most promising cardioprotective target in the last few decades.5
This short article will set the scene for this trial, review the results and conclusions of the study, and then consider why the field of cardioprotection has suffered so many disappointing failures.
The Need for Cardioprotection
Ischaemic heart disease remains the leading non-infective cause of morbidity and mortality in the world. Despite the advances in primary percutaneous coronary intervention (PPCI) during AMI, studies have suggested that up to 50% of the final infarcted territory is viable at the point of reperfusion.6,7 This suggests that continuing injury after, and attributable to, reperfusion occurs in the ischaemic myocardium. A therapy that consistently reduces the infarct size in this setting would have the potential to improve survival and reduce morbidity associated with AMI. IR injury is associated with recurrent ischaemia in the setting of no-reflow syndrome and coronary microvascular injury, heart failure secondary to impaired ventricular function, and an increased scar burden, which is associated with increased arrhythmia. Although timely reperfusion can limit these consequences, many patients are several hours into their AMI at the time of presentation. Mechanisms of myocardial IR injury are addressed in detail in other reviews.8,9
Ischaemic Conditioning
The history of the field of cardioprotection stretches back more than 30 years, with extensive literature that is well reviewed elsewhere.10,11 In summary, ischaemic preconditioning was first demonstrated by Murry et al. when they discovered that a period of transient occlusion, followed reperfusion, of a canine coronary artery reduced infarct size when the same vessel was later subjected to a more prolonged period of ischaemia and reperfusion.12 This effect occurred even when the brief period of IR occurred in a different vascular bed, such as a different coronary artery, or a remote bed, such as a limb.13 This became known as remote ischaemic preconditioning. It was shown to reduce biomarker release and improve long-term outcomes when performed during elective percutaneous coronary intervention.14
Remote ischaemic preconditioning has a number of characteristics that need to be exploited for it to be effective. It is an all or nothing effect, likely reflecting a steep dose–response curve, with a trigger threshold required to achieve protection.15 Both the number of cycles and their duration have been shown to be important in achieving protection.16 In trials where an adequate ‘dose’, usually a number of cycles of ischaemia and reperfusion, has not been achieved, there has been no cardioprotection. Furthermore, the protection is short-lived, with a window of only a few hours during which time the myocardium is protected. A second window of protection caused by transcriptional changes in the nucleus has also been investigated.17–20
Since remote ischaemic preconditioning was difficult to deliver prior to AMI in the clinical setting due to its unpredictable nature, other studies have considered whether postconditioning after the event, or delivering the conditioning stimulus during ischaemia, prior to reperfusion is of benefit. Zhao et al. demonstrated that a postconditioning stimulus, administered in the moments after reperfusion, was effective in reducing infarct size for patients receiving PPCI for AMI.21 This observation suggested that conditioning may be an adjunct to PPCI, limiting the impact of IR injury. While this study used a staggered reperfusion technique, others have demonstrated that RIC could protect the myocardium.22,23
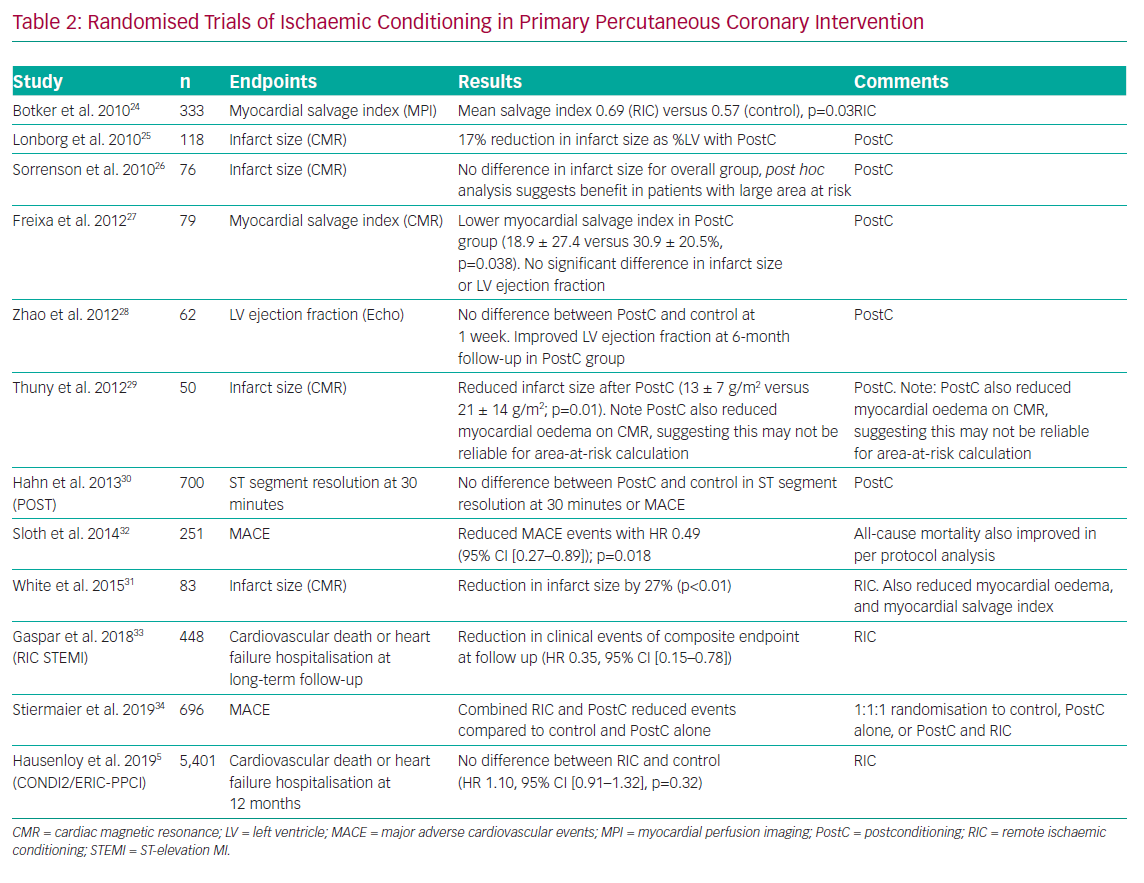
A number of small to medium-sized trials have attempted to confirm that RIC trials have shown benefits on surrogate markers for clinical outcome, such as infarct size and the myocardial salvage index.24–31 Others have confirmed benefits in clinical endpoints, such as major adverse cardiovascular and cerebrovascular events or mortality.32–34 More details regarding these randomised trials of ischaemic conditioning are shown in Table 1. Despite the abundance of small-scale studies demonstrating improvement, the practice of RIC did not translate into a change in clinical practice within interventional cardiology. Ischaemic conditioning in other contexts had been beset by a failure to translate into benefit in the largest-scale trials. For example, the large Remote Ischaemic Preconditioning for Heart Surgery (RIPHeart) and Effect of Remote Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery (ERRICA) trials of ischemic conditioning prior to and during elective cardiac surgery with cardiopulmonary bypass did not show any evidence of improved outcomes.35,36
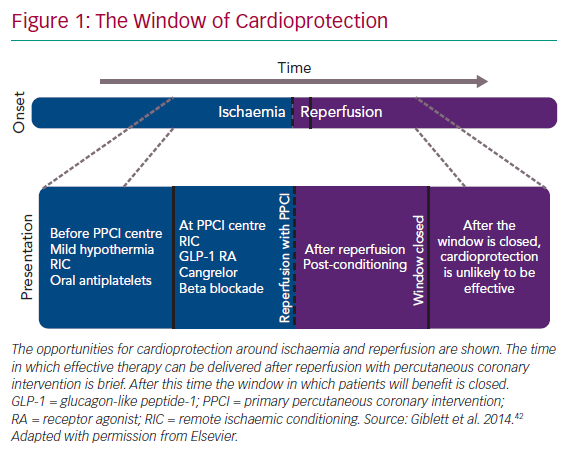
After these studies, a number of editorials suggested that the failures of translation in these studies were at least partially related to the effect of the anaesthetic agent, propofol, on the intracellular pathways of cardioprotection, as shown by Kottenberg et al., together with trial protocols that failed to address the populations most likely to benefit from RIC.37–39 Failure to address the biology of cardioprotection has been behind the difficulty in translating cardioprotective interventions into clinical practice for a long time (Figure 1).1,40–42
The CONDI2/ERIC-PPCI Trial
The CONDI2/ERIC-PPCI trial was a combination of two trials with harmonised protocols to carry out single-blind RIC during ST-elevation MI (STEMI). The trial randomised 5,401 patients at 33 centres to receive either ischaemic conditioning using an automated remote ischaemic conditioning device, or a sham procedure. Some centres offered standard care rather than the sham. The primary endpoint was cardiovascular death or hospitalisation for heart failure at 12 months. Secondary endpoints included major adverse cardiovascular and cerebrovascular events, and infarct size measured using the area under the curve for high-sensitivity troponin T.
The trial found that there were no clinically meaningful differences between the RIC group and the control group. The primary outcome occurred in 8.6% of the control group and 9.4% of the RIC group (treatment effect 1.10, 95% CI [0.91–1.32]; p=0.32) with no significant difference in biomarker-assessed infarct size. The cardiac MRI substudy is still awaited, but regardless of its findings it is unlikely to provide evidence that RIC offers substantial clinical benefit. Other editorial and opinion pieces have concluded that the low overall mortality was the reason for the failure of the study.43 Certainly, the study was appropriately powered for the expected event rate, but one possibility is that the patients were simply not unwell enough to see a significant benefit.
These findings are at odds with those of the previously published investigations discussed above. However, this study was adequately powered to provide a definitive answer, and evaluation of the previous literature now needs to be placed in the context of this study. Other studies were substantially smaller, with low numbers of events, potentially leading to type 1 error. Furthermore, publication bias may have favoured those RIC studies with positive results. The disappointing results of this trial have left researchers in the field looking for alternative targets that can be used to mitigate the impact of IR injury.
Alternative Targets for Cardioprotection
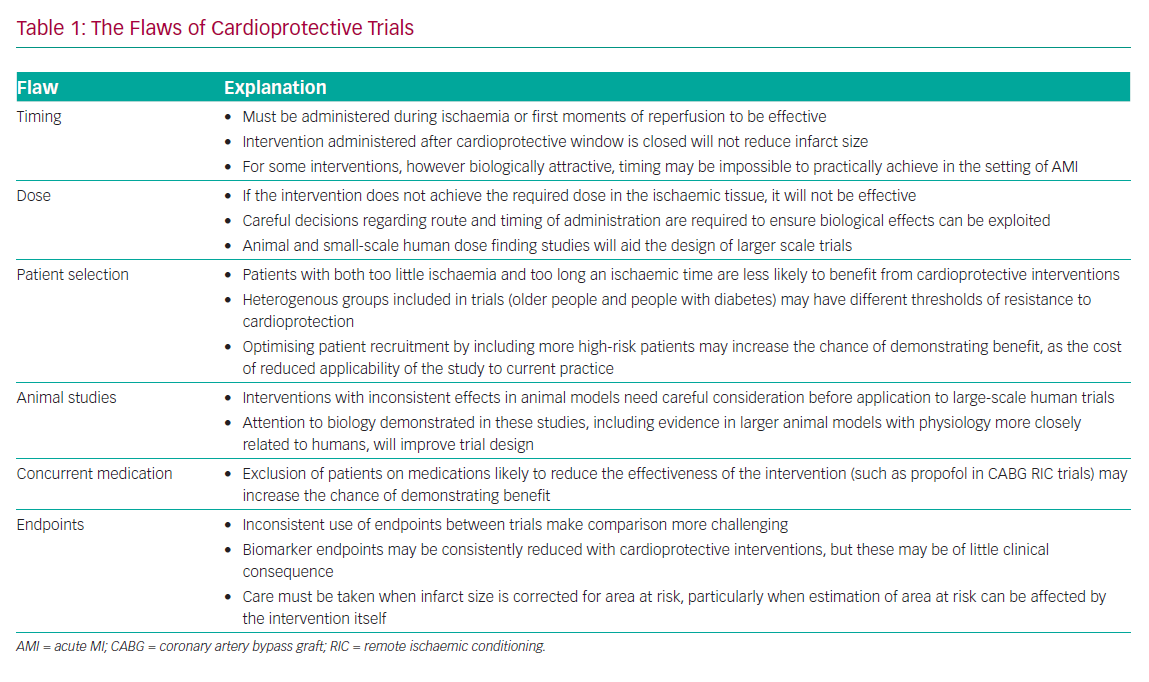
A number of potential pharmacological targets have presented themselves as alternatives to RIC. These include agents that interact with the cascade of biochemical and cellular changes that lead to cell death. A comprehensive review of all targets that have been investigated is beyond the scope of this review, but many of these have failed to translate to clinical practice, because trials have ignored the biology of cardioprotection and benefit in vitro, or small animal studies have not been replicated in human trials. Table 2 offers some of the reasons for the failure of clinical trials.
Adenosine
Binding of the adenosine receptor prior to ischaemia has been shown to provide cardioprotection and reduce infarct size. However, whether this benefit occurs if adenosine is administered after the onset of ischaemia is less clear. While some clinical studies have shown a reduction in infarct size and improvement in clinical outcome with adenosine, others have been neutral.44–47 These trials have been characterised by variability in dose, timing and route of administration. A recent meta-analysis suggests there may be a benefit for intracoronary adenosine in reducing the incidence of heart failure after AMI.48
Glucagon-like Peptide-1
Glucagon-like peptide-1 (GLP-1) is an incretin hormone used as a target in the treatment of type 2 diabetes. GLP-1 receptors may provide cardioprotection through activation of intracellular pathways, such as the reperfusion injury survival kinase and survivor activating factor enhancement pathways.49,50 While these pathways share components with the pathways of ischaemic conditioning, they are not identical.51 GLP-1 receptor agonists have been shown to reduce the frequency of major adverse cardiovascular and cerebrovascular events in high-risk patients with diabetes in some larger cardiovascular outcome studies, improve myocardial function after non-lethal IR injury, as well as reducing infarct size and improving left ventricular function after PPCI in small proof of concept trials.52–57
However, not all trials have shown such benefit. GLP-1 agonists did not reduce the frequency of major adverse cardiovascular and cerebrovascular events following administration to diabetes patients after AMI, nor did they reduce periprocedural MI or cardiac troponin release during elective percutaneous coronary intervention.58,59 This conflicting evidence suggests the need for a larger and more definitive trial to establish benefit
Beta-blockers and Ivabradine
Early administration of beta-blockers has been considered an attractive cardioprotective strategy, since cardiologists are familiar with administering these agents in this patient group, making translation to clinical practice easier if effective. However, there are discrepancies between trials of the beta-blocker metoprolol during STEMI. In the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) study, a large dose of IV metoprolol reduced infarct size when administered before reperfusion, whereas in the Early Beta-blocker Administration Before Reperfusion in Patients with ST-elevation Myocardial Infarction (EARLY-BAMI) trial, a smaller dose given to a less selected population had no effect on infarct size.60,61 Earlier administration to metoprolol was associated with reduced infarct size in a post hoc analysis of METOCARD-CNIC.61 Furthermore, ivabradine, which acts at the sinoatrial node to reduce heart rate, reduced infarct size in a porcine model of IR injury, but when given to humans up to 1 hour after PPCI, no benefit was seen.62,63 This was likely too late to prevent IR injury.
Dose-finding and timing studies are not routinely undertaken in humans in the field of cardioprotection, often leaving more questions than answers in the search for protection. The interpretation of these larger studies has been confounded by the absence of appropriate early studies establishing these characteristics.
Cyclosporine
Cyclosporine A prevents opening the mitochondrial permeability transition pore, which is part of the final pathway of cell death in reperfusion injury.64 Early trials showed apparent benefit in small numbers of patients treated with cyclosporine.65,66 Disappointingly, however, the much larger Cyclosporine to Improve Clinical Outcome in ST-elevation Myocardial Infarction Patients (CIRCUS) trial failed to show any benefit in terms of infarct size or clinical outcomes in patients with STEMI.67 This failure has been attributed to a number of different factors, including an increased length of ischemic time, a difference in the formulation of cyclosporine A and increased use of newer antiplatelet agents.9 Nonetheless, cyclosporine A has fallen at the same stage of translation as RIC. The definitive randomised control trial was neutral in outcome. Other mitochondrial permeability transition pore inhibitors have had mixed or neutral results in small proof of concept trials or animal studies.68 The mitochondrial permeability transition pore may remain a target for cardioprotection with the right agent.
P2Y12 inhibitors
Yang et al. first demonstrated a direct cardioprotective effect with the P2Y12 receptor antagonist cangrelor prior to reperfusion, and showed a 30% reduction in MI size in rabbits.69 Crucially, cangrelor was only effective at limiting MI size if it was administered prior to reperfusion. Cangrelor-mediated cardioprotection (but not platelet inhibition) was abrogated by pharmacological inhibitors of phosphoinositide 3-kinase and mitogen-activated protein kinase kinase 1/2, both known mediators of cardioprotection, suggesting the protective effect of cangrelor was independent of antiplatelet effects.
The cardioprotective effects of P2Y12 inhibitors have also been shown using pretreatment with oral ticagrelor in rats, and cangrelor in primates.70,71 Furthermore, pretreatment with ticagrelor was also shown to reduce MI size in a porcine MI model.72 The Platelet Inhibition to Target Reperfusion Injury (PITRI) trial is currently ongoing, and is evaluating whether cangrelor administered prior to reperfusion would reduce reperfusion injury, as assessed by CMR in 210 patients (NCT03102723).73
Supersaturated Oxygen Therapy
Delivery of supersaturated oxygen with a partial pressure of 750–1,000 mmHg immediately after reperfusion has been shown to reduce anterior myocardial infarct size in the Acute Myocardial Infarction with Hyperoxemic Therapy II (AMIHOT-II) trial.74 Furthermore, the IC-HOT study demonstrated that it was feasible for supersaturated oxygen to be delivered directly to the left main stem during STEMI.75 While the TherOx system has been approved by the Food and Drug Administration for treatment of AMI, randomised trials have only been conducted in relatively small numbers of patients. Similar results have been seen for other cardioprotective interventions, which have proven to be ineffective in larger or less well selected trials. It is worth noting that in AMIHOT-II, only 317 patients out of 2,517 screened with STEMI were enrolled, and most of these failures were due to failure to meet inclusion criteria. It is uncertain how generalisable this data is to the unselected STEMI population. Larger trials in this area are required.
How Can We Do Better with Future Targets for Cardioprotection?
Translation in this field has proved beyond challenging. Despite an enormous investment of time, money and resources in the search for an adjunct to coronary revascularisation to prevent IR injury, no candidate therapy has become a part of the armamentarium of the interventional cardiologist. It is more than 30 years since the discovery of ischaemia preconditioning, but we still wait. Criticisms of decisions taken in trials are not new and have been repeated many times. Nonetheless, it is worth rehearsing some of the challenges of study design, which may provide better direction for future research.
Failure to pay attention to the biology of IR injury, cardioprotection and the specific cardioprotective agents under investigation is a key factor in many studies. No agent will be effective at reducing infarct size if it is delivered too late, or in too small a dose to be effective. Small-scale human trials can provide much information in this regard, and ideally dose and timing studies should be undertaken with appropriately powered surrogates for benefit, before proceeding to larger human studies. Careful selection of patients in both small and large studies is also key to success. Inclusion of patients who will not benefit from the intervention will reduce the power of the study to show benefit. This could include patients with both too little ischaemia or too long an ischaemic time. Enriching the cohort with patients likely to benefit, such as those with anterior infarcts or with moderate ischaemic times, will increase the power of the study to show benefit, although this may come at the cost of generalisability in the real world of clinical practice.
Post hoc analysis of the Botker et al. study suggests that RIC was effective in patients with a delayed transfer to a PPCI centre while not benefiting those who received more rapid reperfusion.24,76 Furthermore, inclusion of older patients, or patients with diabetes, may require adjustment of the dosing regimens to increase the likelihood of benefit.
The selection of a single target has been a consistent theme in trials of cardioprotection. It is possible that an effective reduction in infarct size depends upon accessing multiple pathways of cardioprotection simultaneously.77 There may be additive and synergistic effects between interrelated pathways, or it may be that in a heterogenous population presenting with AMI, different pathways will provide more or less effective cardioprotection for a given individual, depending upon comorbidities and other patient factors. A large number of animal models have been successfully tested, but there have been relatively few human trials of this approach reported. Combination Therapy in Myocardial Infarction (COMBAT-MI) is an example of a clinical trial combining RIC with exenatide infusion to evaluate whether combination therapy is more effective (NCT02404376).
Endpoint selection is a key factor in the success of translational studies.9 Assessment of infarct size using biomarker endpoints, such as cardiac troponin, are very sensitive, but unless high thresholds are used, small changes in these may be of limited clinical relevance, particularly in the context of periprocedural MI.78 Cardiomyocyte-specific creatine kinase has been shown to be the most robust biomarker for quantification of infarct size, but in clinical practice it has largely been replaced with cardiac troponin, which limits its availability for multicentre clinical trials.79 Cardiac MRI is an excellent method to quantify infarct size and assess cardioprotective strategies used during AMI.80,81 The more sensitive myocardial salvage index can be used to increase the power of the study, but remains controversial, as T2-weighted imaging is used to delineate the oedema-based area at risk.82 The myocardial oedema may be impacted by both the timing and nature of the intervention, and the timing of the MRI.29,31
New late gadolinium after AMI is a more robust tool to investigate the impact of cardioprotection in phase II studies.83 Assessment of the microcirculation may also prove to be important. Some retrospective evidence suggests that therapies improving microcirculatory function after MI may improve clinical outcome independently of the infarct size.84 This requires further investigation, and these endpoints investigated prospectively.
Clinical endpoints are important, but should reflect the biology of cardioprotection. Hard endpoints, such as cardiovascular mortality, are important. Endpoints, such as heart failure admission or the need for escalation in heart failure medication, are important, as this is the syndrome most likely to be impacted by therapies that reduce infarct size. They need to be carefully defined, as these are softer clinical endpoints that are vulnerable to a number of biases. Endpoints commonly used in trials of percutaneous coronary intervention strategies and stents, such as the need for repeat revascularisation, reduce the power of these studies to show benefit, as they are unlikely to be affected by the cardioprotective intervention.
Conclusion
The CONDI2/ERIC-PPCI study was a well-run clinical trial of a potentially valuable therapy. Its neutral result leaves behind serious questions for the field of cardioprotection; the next avenue that should be pursued in a large-scale trial is not altogether clear. The development of collaborative groups, such as the European Society of Cardiology Working Group on Cellular Biology of the Heart and the EU-CARDIOPROTECTION COST Action, to push the field forward is welcome, and is likely to produce more fruitful translation to clinical practice.