Exercise right heart catheterisation provides an opportunity to identify haemodynamic phenotypes that reflect a reduction in pulmonary vascular and/or cardiac function, which may not be evident when the patient is at rest. The differentiation of these two pathophenotypes is a key element of exercise right heart catheterisation. Haemodynamic response patterns to exercise may also assist in assessing the severity of valvular heart disease, which can help to improve the management of these conditions.
The objective of this review is to discuss the diagnostic contribution of exercise right heart catheterisation in cardiovascular disease. It will address methodological considerations of exercise right heart catheterisation before discussing ‘normal’ invasive cardiopulmonary responses during exercise – an important contextual framework required for identifying and understanding pathophysiological abnormalities. It will then address the implications of exercise right heart catheterisation to improve diagnosis and treatment in valvular heart diseases.
How to Perform Exercise Right Heart Catheterisation
Exercise right heart catheterisation is more ergonomic when upper body venous access is used to place a multi-lumen pulmonary artery catheter which allows the lower body to be tested using a cycle ergometer. The safety profile of non-invasive exercise stress testing is beyond the scope of this review. However, the relatively small risk of complications associated with right heart catheterisation should be discussed with the patient so they are able to give their informed consent to the procedure.1
Since the majority of complications of right heart catheterisations are related to vascular access, our institution has chosen ultrasound-guided upper extremity venous access as our standard vascular venous access site so we can mitigate those risks, facilitate exercise and potentially improve the patient’s comfort. Once vascular venous access is secured, a balloon-tipped fluid-filled catheter is positioned in the pulmonary artery under fluoroscopic guidance and diagnostic right heart catheterisation is performed at rest.
Heterogeneous procedural practices are encountered for the performance of exercise after initial right heart catheterisation. Supine exercise using a table-mounted ergometer is easy to use, particularly for severely compromised patients but limits ambulatory activity, whereas upright or semi-upright positions avoid this but require the patient to be transferred from the catheterisation table.2,3 In our centre, we have adopted the semi-upright position (30–45º head-up tilt), using a reclining table-cycle ergometer. After supine right heart catheterisation, patients are transferred to the cycle ergometer and pressure transducers are zeroed at the mid-axillary level.4 Since vascular tone and preload conditions change when the upright/semi-upright position is adopted, we suggest that right atrium, pulmonary artery pressures and pulmonary artery wedge pressures (PAWP) and cardiac output (CO) are recorded in the upright/semi-upright position before exercise, and the measurement is performed in the same way at rest as they are during exercise.4,5 Our laboratory uses a submaximal exercise protocol to mitigate interferences in haemodynamic waveforms caused by large respiratory swings. We also use the average pressure measurements from >2 respiratory cycles in preference to end-expiratory measurements.
When measuring CO during exercise, we choose from the thermodilution technique and the direct Fick method, which have both been validated for this purpose and the choice is made by considering operator expertise, exercise protocol in use and equipment availability.6 Arterial blood pressure – measured invasively or non-invasively – and heart rate are also monitored throughout the test.
Normal Cardiopulmonary Haemodynamic Responses to Exercise
Understanding the normal haemodynamic response pattern to exercise in healthy people is particularly relevant for the interpretation of exercise right heart catheterisation findings to discriminate between health and disease phenotypes. The interpretation of haemodynamic responses to exercise needs to take in consideration findings from data derived from studies in healthy people.
Several factors influence the pulmonary and PAWP responses to exercise, which prevents there being single threshold values that define physiologic haemodynamic responses. Ageing is related to higher pulmonary pressure and PAWP during exercise, a phenomenon that is maybe due to age-related increases in ventricular-vascular stiffness.7 Notably, changes in flow are associated with changes in hydraulic pressure modelled on principles of hydraulic pumps.8–11 Therefore, evaluations of pulmonary and PAWP responses to exercise may be best described by pressure-flow relationships. Two pressure-flow relationships that identify the presence of pulmonary hypertension, and the possibility of left heart disease will now be discussed.
The Mean Pulmonary Artery Pressure/Cardiac Output Response to Exercise
Until the 4th World Symposium on Pulmonary Hypertension, which took place in Dana Point, California in 2008, diagnostic criteria for pulmonary hypertension included haemodynamic thresholds both at rest – mean pulmonary artery pressure (MPAP) >25 mmHg – or during exercise (MPAP >30 mmHg).7 Although MPAP normally increases with CO during exercise, lack of uniformity in exercise stimulus and intensity and uncertainty as to the range of physiologic responses made it challenging to establish single threshold values for the diagnosis of disease. At the time, there was increasing evidence to suggest that age is a particular determinant of higher MPAP values in response to exercise, exceeding 30 mmHg in many healthy subjects.12 This prompted the removal of an exercise-induced pulmonary hypertension definition.13 Further prospective data and an additional systematic review including contemporary data from our laboratory confirmed that MPAP either after the onset of exercise or with escalating work rates is higher among older people.12–14
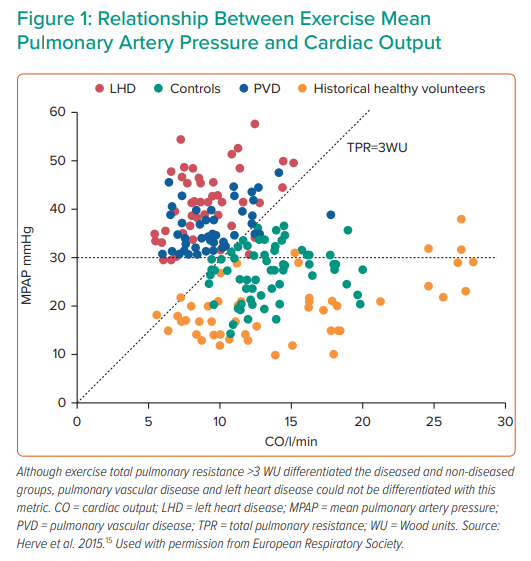
Recent work has refined the understanding of the relationship between increases in MPAP and CO during exercise. Disproportionate increases in MPAP relative to CO augmentation during exercise may represent either a failure of the normal decrease in pulmonary vascular resistance (PVR) with exercise, excessive upstream pressure transmission from the left atrium, or a combination.2,8 Adjusting MPAP values to CO, a variable known as total pulmonary resistance (TPR), is gaining acceptance for detection of abnormalities due to underlying pulmonary vascular or left-heart dysfunction.15,16 Herve et al. demonstrated in a cohort of 169 participants without pulmonary hypertension at rest, that applying a total pulmonary resistance cut-off of 3 WU on exercise holds 96% accuracy to differentiate healthy from deranged phenotypes (Figure 1). Moreover, using such criteria, the positive predictive value for the detection of diseased states was 100%.15 Similar results were found by Kovacs et al., who applied these criteria retrospectively and noted that the false positive rate for healthy subjects decreased from nearly 14% when employing a single point MPAP threshold > 30 mmHg with exercise to less than 3% when employing TPR slope.17
The Pulmonary Artery Pressure/Cardiac Output Response to Exercise
As discussed above for MPAP, recommendations for a single threshold value of the PAWP response to exercise are confounded by the complexity of physiologic responses. PAWP responses are linked to MPAP and are similarly influenced by age and also by exercise duration.7,18 In a study that included 62 healthy subjects divided by age groups, Wolsk et al. showed steeper increases in PAWP with exercise in older people and, importantly, CO augmentation during exercise was attenuated in older subjects compared with younger ones.7 These findings appear to show that higher PAWP responses during exercise are associated with physiologic ageing of the cardiac chamber function. Prospective data from our laboratory demonstrate that PAWP may increase precipitously shortly after the onset of exercise in older adults, with subsequent decline in values as exercise is sustained over minutes, reinforcing the importance of considering exercise duration for the appraisal of PAWP response to exercise.12,18 Systematic reviews performed by our laboratory and by Kovacs et al. further endorse the association of age with higher PAWP responses during exercise, and can exceed 20 mmHg and even 25 mmHg both previously suggested cut-off values for the detection of heart failure with preserved ejection fraction (HFpEF) during exercise right heart catheterisation.7,12,14,18,19
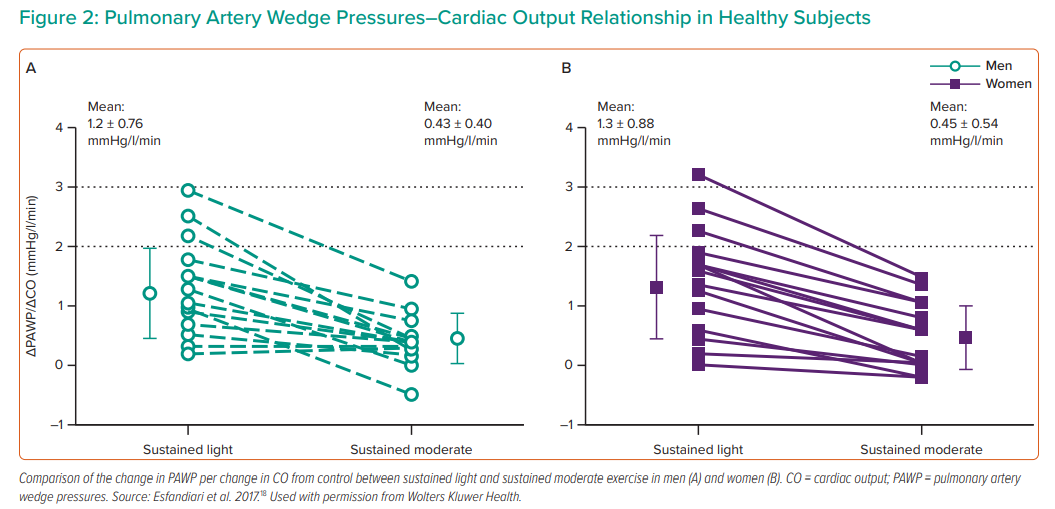
The challenges of identifying a single upper limit threshold have prompted evaluation of the PAWP/CO relationship as a more comprehensive differentiation between physiologic and pathologic PAWP responses to exercise. In a study that included exercise right heart catheterisation among 36 healthy volunteers, our laboratory demonstrated that the slope between PAWP/CO does not exceed 2 mmHg/l/min during submaximal exercise performance.18 Importantly, in order to obtain steady-state flow measurements, CO measurements were performed after 3 minutes of exercise at fixed work rates (Figure 2). Despite a disparate exercise protocol, our findings were replicated in a study from Eisman et al. in which maximal exercise tests with incremental ramp protocols were used: exercise PAWP/CO slope ≤2 mmHg/l/min differentiated controls from those with HFpEF and identified patients with improved event-free survival from heart failure.20 This study also demonstrated greater exercise capacity and ventilatory efficiency in people with a lower PAWP/CO slope.
Interpretation and Indications for Exercise Right Heart Catheterisation
Right heart catheterisation is recommended to confirm the diagnosis of pulmonary hypertension to support treatment decisions. Clinical scenarios include the diagnosis of pulmonary arterial hypertension (PAH) for which pulmonary vasodilator therapy may be prescribed, to assess the risk of graft failure in patients with left heart disease undergoing evaluation of candidacy for cardiac transplantation, and to assess the severity of structural heart disease such as congenital cardiac shunts, or valvular heart lesions, particularly when non-invasive imaging is inconclusive.
Pursuing a diagnosis of PAH illustrates some of the limitations of haemodynamic assessment in the resting supine state. Identifying patients who are eligible for treatment depends on showing evidence of pre-capillary pulmonary hypertension, defined as MPAP >20 mmHg and PAWP <15 mmHg and PVR >3 WU. However, the current population of patients with PAH is older than previously considered and a proportion has a high prevalence of cardiovascular risk factors.21 As such, this population will overlap with patients at risk of abnormal left heart filling pressures due to left ventricular (LV) and systemic vascular stiffening and LV diastolic impairment. It may be challenging to differentiate pre-capillary pulmonary hypertension from post-capillary pulmonary hypertension, also known as pulmonary hypertension, related to left heart disease (PH-LHD). Differentiating PAH from PH-LHD is essential as specific therapy improves clinical outcomes in PAH but is potentially deleterious in PH-LHD.22–27
Borlaug et al. and others have demonstrated the ability of exercise right heart catheterisation to identify patients with dyspnoea and normal resting haemodynamics to reveal abnormal PAWP responses, employing point measurements that exceed threshold values. Among patients with PH present at rest, exercise may similarly reveal or clarify the dominant mechanisms contributing to PH. In our current practice, pressure-flow relationships are considered for the MPAP and the PAWP. The combination of an abnormal MPAP/CO relationship (>3 mmHg/l/min) and a PAWP/CO slope <2 mmHg/l/min suggests that the central derangement lies at the level of the pulmonary vasculature, which can be helpful when the PAWP at rest is in a borderline range. Alternatively, concordantly abnormal behaviour of both the PAWP/CO slope (>2 mmHg/l/min) and the MPAP/CO relationship may suggest that elevated pulmonary pressures are entirely driven by left heart filling pressures. In our pulmonary hypertension programme, exercise right heart catheterisation can support clinical decisions regarding administration of pulmonary vasodilator therapy. However, several questions remain unanswered and future research is directed at understanding whether decision-making based on exercise right heart catheterisation affects clinical outcomes or predicts a response to treatment. Another area of research relates to whether patterns of MPAP and PAWP based on exercise changes in CO may elucidate the contribution of pulmonary vascular disease in the presence of PH-LHD.
Rationale for the Use of Exercise Right Heart Catheterisation Before Valvular Interventions
The field of valvular heart diseases has substantially evolved in recent years, with tremendous progress in percutaneous interventional techniques for valvular heart diseases. As the indications for percutaneous interventions expand to populations with moderate symptoms, it may be useful to have additional evaluations that may help to select candidates most likely to benefit from the procedure. In this setting, exercise right heart catheterisation may objectively unmask the occurrence of symptoms and underlying haemodynamic abnormalities, particularly in patients with vague, non-specific symptoms and in those in which the correlation between decreased functional capacity and the severity of underlying valvular heart disease assessed by echocardiography is unclear. The available data on exercise right heart catheterisation applications in heart valve diseases is summarised in Table 1. Currently, there is remarkably limited data on the role of exercise right heart catheterisation in the setting of aortic regurgitation, pulmonic valve disease or tricuspid stenosis and these topics are not addressed.
Aortic Stenosis
Aortic stenosis (AS) affects one in 20 people aged over 65 years, the prevalence of AS increases with age and it is currently the most common primary valve disease leading to surgical or transcatheter intervention in Europe and North America.28,29 The prognosis of AS is negatively affected by the presence of symptoms and aortic valve replacement (AVR) is indicated once symptoms occur.29,30 Although up to 50% of patients with severe AS claim to be asymptomatic at diagnosis, patients may unconsciously adapt their lifestyle by reducing their activity levels or attributing exercise intolerance to other reasons, such as ageing or non-cardiac comorbidities.31,32 Hence, symptom status assessment in patients with severe AS is challenging. In addition, about 30% of patients with moderate AS have exercise intolerance and the role of AS in this exercise limitation needs careful examination.33
Furthermore, the assessment of AS severity relies on the evaluation of aortic valve area (AVA) and transvalvular pressure gradients. Currently, AS is considered severe when AVA is ≤ 1 cm2 and aortic jet velocity is >4 m/s or the mean transvalvular gradient is ≥40 mmHg.29,30 Although AVA is theoretically the ideal determinant of AS severity, AVA may be underestimated in low-flow states, such as in the presence of reduced ventricular systolic function (LVEF <50%) or in patients with preserved LVEF and low antegrade flow, such as small left ventricular cavity, marked left ventricular hypertrophy or significant mitral regurgitation.34–37 Therefore, comprehensive assessment of AS severity is extremely relevant and there is growing interest on the use of provocative manoeuvres for this purpose.
Currently, the 2017 European Society of Cardiology (ESC)/European Association for Cardiothoracic Surgery (EACTS) guidelines for the management of valvular heart disease recommend surgical aortic valve replacement (SAVR) for patients with severe AS in which symptoms are unmasked (Class I, level of evidence [LOE] C) or in those with a fall in blood pressure (Class IIa, LOE C) during exercise testing. SAVR is also recommended (Class IIa, LOE C) in patients with pulmonary hypertension (systolic pulmonary pressure >60 mmHg).29 However, the discrimination between AS-related symptoms versus deconditioning, frailty, or other causes of dyspnoea on exertion is problematic in a traditional exercise test and invasive documentation of haemodynamic derangement might be particularly helpful.
In this scenario, Lancellotti et al. demonstrated in 105 patients with severe asymptomatic AS undergoing stress echocardiography that the development of exercise-reduced pulmonary hypertension observed in 55% of these cases is associated with a twofold increase in the incidence of adverse cardiac events. Exercise-induced pulmonary hypertension defined in this study as estimated pulmonary artery systolic pressure >60 mmHg during exercise was derived from regurgitant jet velocity of the tricuspid valve. Thirty-five cases were excluded because of the absence of measurable pulmonary artery systolic pressure during exercise, potentially preventing the use of non-invasive assessment of exercise-induced pulmonary hypertension in this patient population.38 Our laboratory and van Riel et al. have demonstrated development of flow-related pressure gradients between the right ventricle and the pulmonary artery during exercise.39,40 As such, the assumption that calculated right ventricular systolic pressure (the sum of right atrial pressure and transtricuspid pressure gradient, derived from regurgitant jet velocity of tricuspid regurgitation) is an accurate surrogate of pulmonary artery systolic pressure during exercise may be flawed. Hence, in patients with non-diagnostic stress echocardiography, exercise right heart catheterisation may be considered.
In a population of 33 patients with asymptomatic moderate or severe AS who underwent exercise right heart catheterisation, lower values of pulmonary artery oxygen saturation at peak exercise were associated with worse clinical outcomes at the 2-year follow-up period.41 Although this finding may point towards an association between poor outcomes and attenuation of CO increase during exercise in this population, the lack of CO measurements during exercise precludes definitive conclusion in this aspect and this hypothesis needs to be further addressed. In this setting, in a cohort of 39 patients with severe asymptomatic AS, Christensen et al. demonstrated a positive correlation between left atrium dilatation and both PAWP and MPAP at rest and during exercise. Notably, remarkably elevated PAWP during exercise was observed in 85% of these cases, despite normal PAWP at rest in about 50% of the population. Importantly, changes in MPAP and PAWP during exercise were associated with increased risk of a composite outcome of aortic valve replacement, unplanned hospitalisation or death.42
Accordingly, exercise right heart catheterisation might further refine the understanding of potential contributions of AS in the genesis of haemodynamic derangements by putting in perspective the behaviour of pulmonary vasculature and left-sided filling pressure responses in relation to flow responses. The prognostic implication of haemodynamic abnormalities disclosed during exercise in this population is still not fully explored and there is tremendous opportunity for future research in this field.
Mitral Valve Diseases
Mitral Stenosis
Mitral stenosis is the only valvular heart lesion that is still mostly caused by rheumatic heart disease in developed countries and despite the fact that the prevalence of degenerative calcific mitral stenosis has increased as the population has aged, the natural history of the latter is not clearly understood and significant non-rheumatic mitral stenosis is rare.43,44 The prognostic and symptomatic burden of mitral stenosis is strongly related to the haemodynamic consequences of transmitral gradients and development of pulmonary hypertension.45
Resting measurements might underestimate the functional haemodynamic consequences of mitral stenosis and exercise testing is recommended when discrepancies between mitral stenosis severity and symptoms are encountered.29,30 In symptomatic patients with severe mitral valve stenosis, mitral valve interventions are recommended, preferably by percutaneous mitral valve commissurotomy.29,30 Furthermore, in order to reduce the negative consequences of mitral stenosis, percutaneous mitral commissurotomy is recommended in asymptomatic patients with pulmonary hypertension or with other features that might suggest higher risk of haemodynamic decompensation. Haemodynamic features of pre-capillary pulmonary hypertension do not correlate with left atrial pressure values at rest and the severity of mitral stenosis is not a reliable predictor of pulmonary pressures.46
In a study of 53 ambulatory patients with mitral stenosis, Reis et al. demonstrated that mean diastolic mitral valve gradient at peak dobutamine stress echocardiography ≥18 mmHg predicted cardiovascular hospitalisation and the incremental prognostic power of dobutamine stress echocardiography was most pronounced among patients with a mitral valve area between >1 cm2 and ≤1.5 cm2.47 However, this study included mostly symptomatic patients and thus the prognostic role of dobutamine stress echocardiography in asymptomatic patients with mitral stenosis remains unclear. In patients with discrepancies between symptoms and mitral stenosis severity, resting indexes are not able to identify poor exercise capacity or the development of severe pulmonary hypertension at exercise.48
The use of combined exercise right heart catheterisation and Doppler echocardiography in the assessment of mitral stenosis was demonstrated more than two decades ago. Through assessment of transvalvular mitral valve flow, assessed by the thermodilution technique, divided by mean transmitral flow velocity, Voelker et al. challenged the dogma that stenotic mitral valve area would be fixed during exercise.49 Two subgroups were identified: those with mitral valve reserve defined by an increase in mitral valve area >20% and those without (<20% increase in mitral valve area at exercise). Despite similar mitral valve area at rest and similar change in mean transmitral flow velocity during exercise, patients with mitral valve reserve demonstrated greater increases in CO and transvalvular flow. Also, whereas exercise resulted in stroke volume augmentation among those with mitral valve reserve, stroke volume decreased during exercise in patients without mitral valve reserve. Therefore, CO augmentation was driven exclusively by increases in heart rate in those without mitral valve reserve. The exercise effective mitral valve area <1.2 cm2, calculated according to the method described above, had higher sensitivity and specificity to detect severe mitral stenosis than other haemodynamic measurements.
In summary, in the setting of mitral stenosis, the correlation between resting indexes and decreased exercise capacity or severe haemodynamic derangements at rest or during exercise is poor. Although, exercise right heart catheterisation, preferably combined with Doppler echocardiography, might provide valuable incremental information on the haemodynamic repercussions of mitral stenosis, the clinical usefulness and prognostic implication of exercise haemodynamic findings remain to be determined.
Mitral Regurgitation
Mitral regurgitation (MR) is the most prevalent valvular disease in developed nations and it is the second most common cause for cardiac valve surgery in Europe.43,50 Whereas primary MR is related to derangements at the mitral valve apparatus level, secondary (also known as functional) MR is most frequently related to imbalance between closing and tethering forces on the mitral valve as a result of altered left ventricular geometry. Indications for intervention differ between primary and secondary MR and will be discussed separately.
Primary Mitral Regurgitation
In primary MR, symptoms usually occur first during exercise and the onset of symptoms represents a key landmark in the course of disease. Symptom onset may be insidious and decreased functional capacity might be unappreciated in chronic MR. The correlation between exercise haemodynamic derangements and the contribution of MR might be particularly informative in the setting of chronic MR.
Currently, the ESC/EACTS guidelines encourage the use of exercise to determine functional capacity in asymptomatic patients with primary MR, with particular attention to significant increases in systolic pulmonary arterial pressure.29 Similarly, the American Heart Association/American College of Cardiology guidelines also support the use of exercise haemodynamic assessment when discrepancies between MR severity and symptoms are observed or when the degrees of LA and/or LV remodelling are apparently disproportionate to the degree of MR (Class IIa, LOE B).30
In asymptomatic patients with chronic primary MR, remarkably elevated systolic pulmonary artery pressures (SPAP >60 mmHg), observed in 46% of patients in this study, were associated with worse prognosis in up to 2-year follow-up.51 Also, despite overall unremarkable haemodynamic findings at rest, SPAP >60 mmHg was observed in one-third of asymptomatic patients with chronic primary MR in a study that included symptomatic and asymptomatic patients with chronic primary MR. Peak oxygen consumption was not different between these two groups, reinforcing the potential relevance of comprehensive assessment of exercise haemodynamic parameters.52
Therefore, in the rapidly evolving field of surgical and percutaneous interventions for the management of primary MR, exercise right heart catheterisation might further refine the assessment of asymptomatic patients with underlying exercise-induced pulmonary hypertension and a worse prognosis and it might also be particularly helpful in clarifying discrepancies between MR severity and the presence of symptoms.
Secondary Mitral Regurgitation
Secondary MR most frequently reflects altered closing and tethering forces on the mitral valve as a result of altered LV geometry. Secondary MR has been strongly related to decreased quality of life, increased rate of hospitalisation for congestive heart failure and increased mortality.53,54 During exercise, increasing the degree of functional MR leads to attenuation of increase in forward stroke volume and increased backward flow results in a disproportionate increase in left atrial and pulmonary pressures, therefore increasing the pulsatile load in the pulmonary circulation.55 Worsening of MR during exercise has been associated with impaired exercise capacity, even when MR is modest at rest.56
The results of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial, in which transcatheter treatment of significant secondary MR was associated with a 47% risk reduction in the occurrence of hospitalisation for heart failure within 24 months follow-up, as well as significant mortality rate reduction and improved quality of life and functional capacity have changed the landscape in the field of secondary MR treatment; previously considered a marker of advanced heart failure, secondary MR is currently acknowledged as a potential target for optimal treatment of heart failure.57
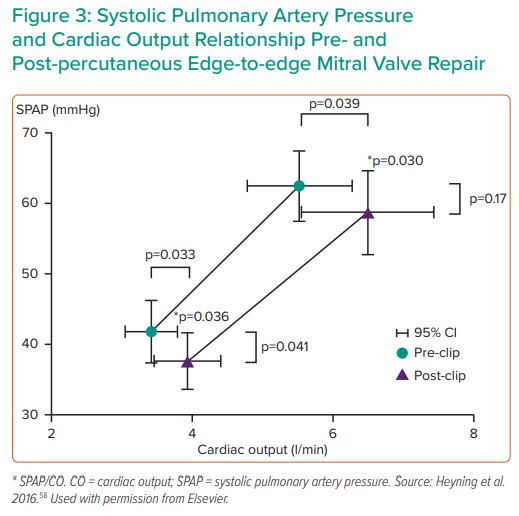
Percutaneous edge-to-edge mitral valve repair resulted in an improved exercise haemodynamic profile in a small study, reinforcing the pathophysiological relevance of treating secondary MR (Figure 3).58 Although the concept of using invasive haemodynamic assessment as a strategy to identify responders to mitral valve interventions in the setting of secondary MR might hold promise, data to support such a strategy is still scant and future research should address this hypothesis.
Tricuspid Regurgitation
Tricuspid regurgitation is most commonly caused by tricuspid valve annular dilatation and/or right ventricle enlargement and is also known as functional tricuspid regurgitation. It is associated with a worsened prognosis and decreased functional capacity, regardless of left-sided heart disease or pulmonary hypertension.59,60 Recent developments in the field of transcatheter tricuspid valve interventions have changed the landscape in the management of functional tricuspid regurgitation. However, there is a lack of understanding on the subgroups of patients that could benefit from transcatheter tricuspid valve interventions and most cases are performed on a compassionate basis.61
Functional tricuspid regurgitation is often associated with other cardiovascular diseases, such as AF, heart failure with both preserved and reduced ejection fraction, MR or pulmonary hypertension and these comorbid conditions may confound the assessment of tricuspid regurgitation’s contribution to the pathogenesis of decreased functional capacity in this scenario. In a study that included invasive haemodynamic assessment during exercise, mismatch between CO and metabolic needs have been demonstrated in the setting of severe functional tricuspid regurgitation. This study also demonstrated abnormal PAWP response to exercise in a population of patients with isolated tricuspid regurgitation and without left-sided heart disease, raising the possibility that pulmonary venous congestion might contribute to exercise intolerance in this patient population. Also, despite abnormally increased PAWP, LV transmural pressure substantially decreased during exercise, indicating reduced LV preload at exercise, potentially driven by the occurrence of right ventricular dilatation and consequent direct ventricular interaction during exercise.62
Exercise right heart catheterisation may present the opportunity to thoroughly assess right ventricular systolic function and right ventricular contractile reserve, which may be relevant for the selection of patients who might benefit from transcatheter tricuspid valve interventions, refining the indications for the use of this evolving technology.63,64 Alternatively, there are limited tools to identify patients who are less likely to benefit from transcatheter tricuspid valve interventions, as patients with pulmonary arterial hypertension and severe tricuspid regurgitation have poor survival rates.65 Thus, demonstration of remarkable pre-capillary exercise pulmonary hypertension haemodynamic phenotype might represent a subgroup of patients in whom tricuspid valve interventions may not be beneficial for the primary haemodynamic abnormality. However, there is lack of consensus on how to perform such assessments and data in this setting is still scarce, limiting the clinical applicability of exercise right heart catheterisation in this specific scenario.
Conclusion
Exercise right heart catheterisation may objectively unmask symptoms and underlying haemodynamic abnormalities that are not evident at rest. Furthermore, it may facilitate the distinction between pre- and post-capillary pulmonary hypertension. As the field of transcatheter interventions for valvular heart disease continues to grow, the potential for exercise haemodynamic evaluation to provide physiologic insights and possibly provide clinical decision support should be further explored.