During the last two decades, transcatheter aortic valve replacement (TAVR) has evolved into an established treatment in patients with severe aortic stenosis and a suitable alternative in patients with pure aortic regurgitation deemed unsuitable for a surgical approach.1 During recent years, it has become evident that an optimised implantation depth (OID) is crucial to obtain the best haemodynamic and clinical outcome. For instance, a transcatheter heart valve (THV) implantation located too high might result in coronary obstruction, paravalvular leak (PVL), or valve embolisation. In contrast, deep implantation toward the left ventricular outflow tract (LVOT) might predominantly result in an increased risk for conduction disturbances, impairment of the mitral valve function, and also PVL. Thus, OID ensures stable anchoring, a better haemodynamic profile and is associated with a potential reduction in permanent pacemaker implantation (PPI; which continues to range between 15% and 35% using self-expanding devices and is one of the last remaining problems of the current treatment era).2–6 The aim of this review is to outline factors that might influence final implantation depth (ID) and to provide tools to achieve an OID during TAVR procedures, potentially influencing the outcome.
Membranous Septum Length
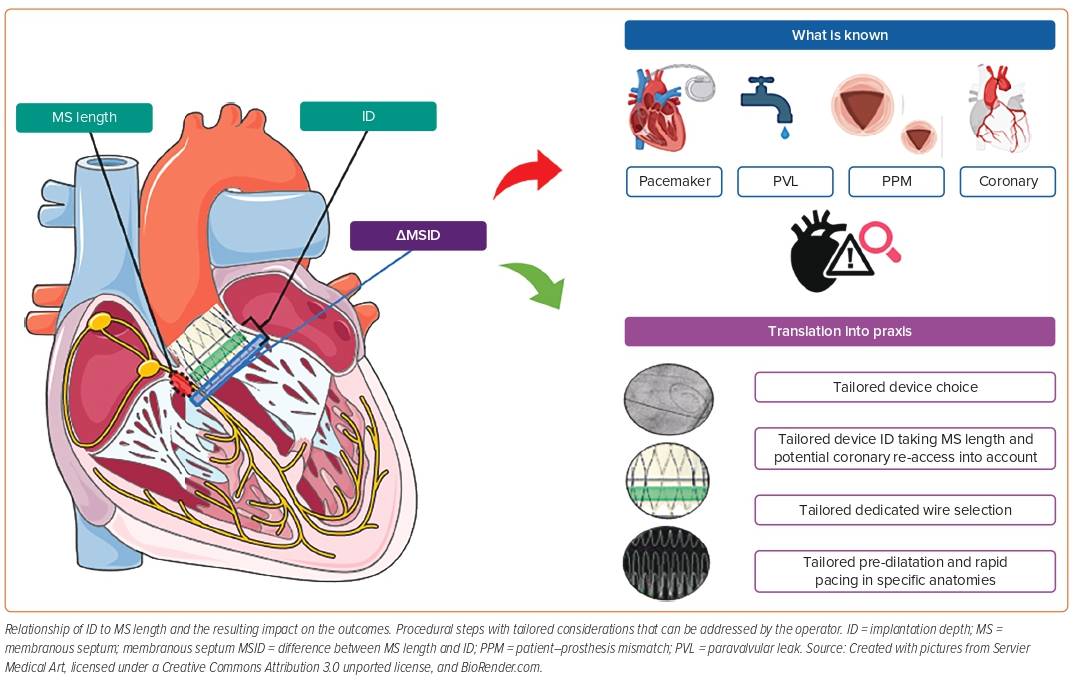
The membranous septum (MS) is the fibrous component of the interventricular septum lying at the base of the interleaflet fibrous triangle separating the right and non-coronary sinuses of the aortic valve. The atrioventricular (AV) node is located in this triangle and continues as the AV His bundle through the lower border of the MS. However, there is considerable variability in MS length and its anatomical relationship with the AV node and the His bundle.7 The left bundle branch emerges into the interventricular septum from beneath the MS close to the base of the interleaflet triangle. This relationship explains why a left bundle branch block (LBBB) is the most common conduction disturbance following TAVR. Several studies have reported an association between device ID, MS length and PPI.2,8,9 Jilaihawi et al. described a patient-specific approach called minimising depth according to the membranous septum (MIDAS), and reported very low and predictable rates of PPI compared with previously published data, which is expected to be a game changer regarding THV implantation strategy.2 The working group proposed patient-specific best practice for device positioning, aiming for a device implantation depth according to the non-coronary cusp below the MS length (ID < MS length), also expressed as a ratio.2 However, micro- and macro-movements of self-expandable devices happen predominantly during the final release, and for this reason, the selected ID must not be too high. In this context, it must also be stated that the relationship between ID and MS length should be interpreted with caution because angiography tends to underestimate ID measurements compared with multislice CT.3,10–12
Consequently, the manufacturer of the self-expandable THV Evolut platform (Medtronic) introduced new best practice recommendations for valve deployment in 2020, including the cusp-overlap technique (COT) to reach a higher ID (target ID 3 mm instead of 3–5 mm toward the LVOT). The advantage of a COT view lies, among other things, in an elongation of the LVOT with resolution of the delivery catheter parallax and a consecutive accentuation of the non-coronary and right coronary commissures.13,14 Application of the COT was shown to be associated with significantly lower PPI rates in several studies and current meta-analyses.15–21 Furthermore, the symmetry of the implanted THV might be improved because of antegrade positioning, better visualisation of the non-coronary cusp nadir and accurate assessment of the actual device depth without foreshortening of the LVOT.17 Even if the COT was established for specific self-expandable valves due to their greater extension into the LVOT, it has already been shown how this technique might influence ID, PPI need, and haemodynamic performance using balloon-expandable and other devices.22,23 However, according to current data, there is no evidence for a potentially increased risk of coronary obstruction or upward dislocations (pop-outs) with a higher ID (within appropriate limits).
Anatomical Conditions
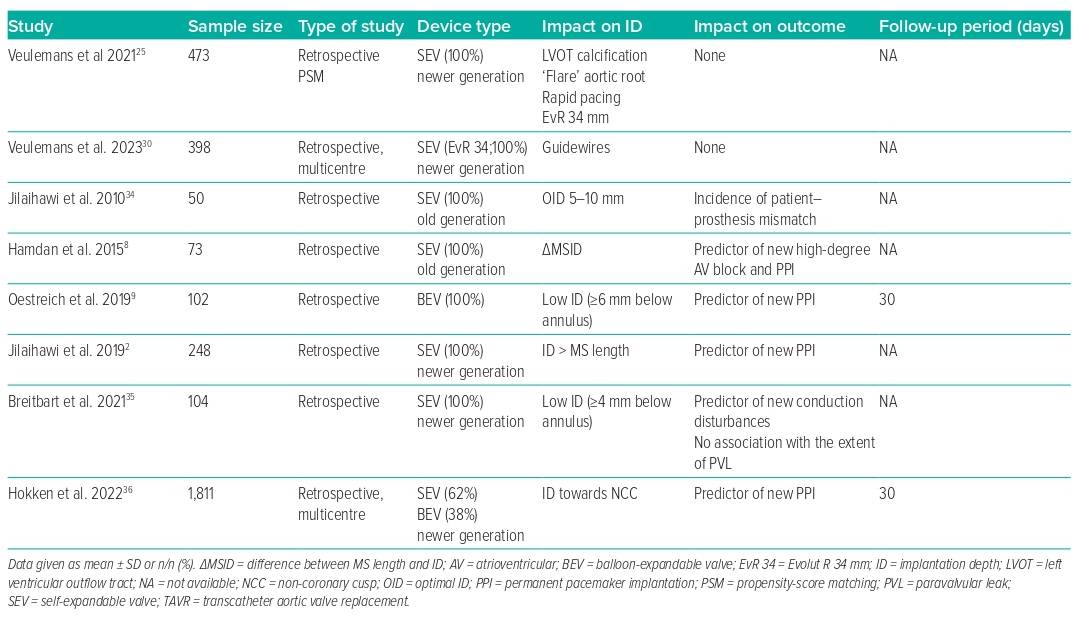
Specific anatomical conditions might be challenging in the stable and haemodynamically favourable deployment of the THV. Upward migration of the THV is usually observed in patients with left ventricular hypertrophy with a septal bulge and a heavily calcified aortic valve. In contrast, downward migration is known to occur in the case of a large LVOT and less calcified aortic valves.10,11,24,25 The final device position may also be influenced by geometrical aspects of the configuration of the aortic root. A flared configuration, in which the LVOT is smaller than the annulus, leads mainly to an upward direction, while a tube or tapered configuration results in a deeper ID.25 Furthermore, an unfavourable angulation of the aortic root and thoracic or abdominal aortic tortuosities may lead to enhanced shear forces on the delivery system, resulting in delayed transmission of manipulations to achieve the correct anatomic position and, hence, dislocation primarily toward the LVOT. In this context, LVOT calcification may inhibit micro- and macro-movements of the device toward the LVOT and enable a higher ID. However, the opposite can also be observed when pronounced mitral annular calcification leads to a slipping effect toward the LVOT as a result of increased pressure on the device.
Device Choice and Dilatation Processes
In general, there are huge differences between the available devices. We discuss here only the leading balloon- and self-expandable devices on the market. Concerning balloon-expandable valves, the ID seems more predictable, and the risk of PVL is considerably less. In the context of self-expandable devices, the ID seems more crucial, given that self-expandable THVs extend further into the LVOT. As an advantage, the Evolut THV provides the ability to re-sheathe and re-capture the device to reach a gradual, controlled and precise deployment in the region of interest. Moreover, the impact of pre-dilatation is a frequently discussed topic.26,27 However, there is no evidence that pre-dilatation favours dislodgement, a regularly higher ID, or a higher risk for PPI according to ‘first hit on the conduction system’ philosophies.11,25–29 However, pre-dilatation may result in severe regurgitation, especially in bicuspid anatomies, requiring fast implantation procedures with careful catheter handling to avoid dislocation during deployment. Regarding post-dilatation, rapid ventricular pacing should be stopped only after the deflation of the balloon to avoid a potential dislocation of the prosthesis due to the recovering stroke volume.
Wire Choice
Transcatheter aortic valve implantation (TAVI) guidewires are necessary to support the THV deployment process. They guide the device through the iliofemoral arteries and the aorta and provide support during the native valve crossing. However, some anatomical conditions might be challenging during the deployment process in terms of device stabilisation and target OID. The profiles of commercially available pre-shaped guidewires differ in stiffness and stabilisation ability and may influence the implantation process. Each guidewire has specific support features for different levels of the aortic root, which would probably influence the stabilisation of THV deployment and the resulting ID. Our group provided the first structured evaluation of the procedural impact of different guidewires in a large cohort of all-comer TAVI patients, demonstrating that the use of stiffer guidewires might result in a higher ID when treating larger anatomies with self-expandable valves.30 However, there was no impact of guidewire choice on 30-day outcomes, including conduction disturbances and pacemaker need. Identification of potential benefits regarding the outcome requires further investigation.
Mode of Pacing
Fast and rapid ventricular pacing (RP) manoeuvres temporarily reduce cardiac output, thus enabling stable valve deployment. Fast pacing is usually defined as an episode of ventricular pacing between 100 and 160 BPM to reach a systolic blood pressure <100 mmHg during the final valve release. RP involves higher frequencies of approximately 200 BPM to entirely inhibit cardiac output during the final valve release, which is crucial for the safe positioning of rapid-deployment balloon-expandable THV devices. However, RP might also be useful for the deployment of self-expandable devices in the case of specific anatomical conditions. For instance, fast pacing alone is sometimes insufficient to reduce cardiac output to a stable level. We have shown for the first time that RP might be helpful in reaching a higher ID using self-expanding devices in specific anatomies.25
Possible Effects of ID on Outcomes and Mortality
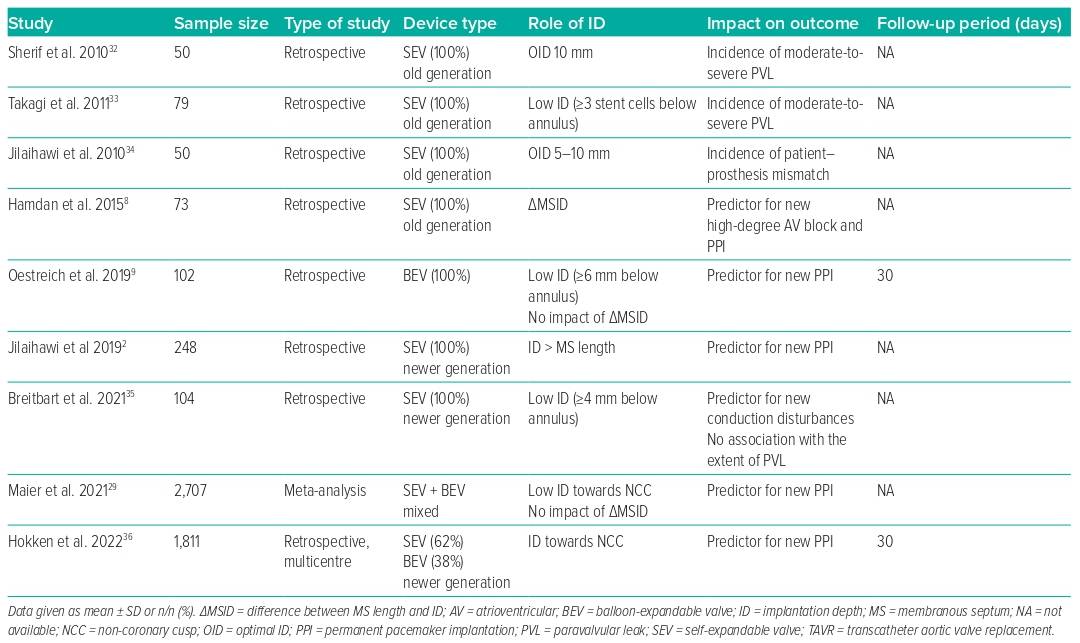
Given that the ID influences the rate of PVL, PPI and the likelihood of coronary re-access, this may also affect long-term outcomes following TAVR. These considerations have become more relevant in the context of treating younger and lower-risk patients.31 The risk of conduction disturbances, consecutive PPI need, and the risk of PVL depend on the interaction of the radial forces of the device-specific stent frame with the calcification distribution. We summarise in Table 1 the impact of the final ID on outcomes and in Table 2 all of the variables that may influence ID and the associated outcomes when available. However, long-term data are rare.
Influence on PVL and Outcomes
The impact of PVL gained increasing attention in the last decade after greater than mild PVL was reported with impaired short- and long-term outcomes.37,38 Sherif et al. were one of the first groups to provide a detailed analysis of the effects of ID on the native annulus in terms of risk of PVL with the first-generation self-expandable CoreValve system (Medtronic).32 They showed that the OID with the older device was approximately 10 mm toward the LVOT and was strongly dependent on the LVOT-to-aortic angle. Takagi et al. stated that a too-deep ID was associated with a threefold increased risk of moderate-to-severe PVL.33 Jilaihawi et al. also underlined the role of the ID in avoiding patient–prosthesis mismatch, defining an OID of 5–10 mm below the native non-coronary cusp.34 However, studies on newer generation THV devices and implantation techniques show no influence of the ID on the grade of PVL, which is currently considered to be very low.35 This might be due to the fact that newer generation devices are generally designed to be either repositionable and/or to have a better sealing capacity.
Influence on PPI and Outcomes
As noted earlier, there seems to be a strong correlation between MS length and ID in terms of conduction disturbances and need for PPI, one of the remaining issues in outcome following TAVR.2,39 Moreover, increasing evidence indicates that PPI need is associated with poor outcomes.40 However, the existing data regarding the impact on mortality are still heterogeneous.41
In this context, Hokken et al. confirmed that MS length was an independent predictor of PPI across different THV platforms, identifying three risk groups by MS length: MS ≤3 mm was defined as high-risk for PPI with an incidence of 20%, while MS length >7 mm was defined as low risk (<10%).36 However, an ID less than the MS length was associated with a lower PPI rate after TAVR with the balloon-expandable Sapien 3 (Edwards Lifesciences) or self-expandable Evolut THV platform. Nevertheless, not only the device platform but also larger device sizes were shown to be associated with higher rates of PPI, while the degree of oversizing is usually unrelated.2 The largest Evolut THV device was shown to be an independent predictor for PPI, even when adjusting for an appropriate pre-release ID according to the MS length, suggesting device-specific factors. We confirmed that this large self-expandable device was an independent predictor for a deep ID and that most of the previously reported determinants, such as MS length and ID, failed to predict PPI for this device.4,25 While the inflow of the smaller Evolut devices is cylindrical, the largest device is almost conical, potentially leading to unpredictable post-release effects and mismatch of the intended ID. Importantly, this device exerts a strong force to expand and has a broad annular contact area, which is outstanding in this context and might independently have an impact on the risk of permanent conduction disturbances.
Influence on Coronary Re-access and Outcomes
Even if the incidence of acute coronary syndrome following TAVR remains low, multiple studies have shown difficulties in coronary re-access when the THV commissural suture posts are placed in front of the coronary ostia.42–44
A prescient implantation technique, including commissural and/or coronary alignment, is currently considered as optimal lifetime management, especially in younger patients with pre-existing coronary artery disease and a higher probability of future coronary (re-)intervention.45 A selected ID that is too high may impede coronary re-access in specific anatomies, although commissural/coronary alignment provides appropriate prevention strategies.
Figure 1 provides an overview of the interaction of ID and MS length, the impact on the outcome, and which procedural steps can be modified by the operator to obtain an appropriate intra-procedural result.
Conclusion
TAVR has evolved into an established treatment in patients with severe aortic stenosis and is now being used for younger patients. Although optimisation of THV devices and increasing treatment experience led to improved outcomes, some issues, such as PPI need and PVL, remain. Further refinements in the THV deployment process are crucial to obtain the best functional and haemodynamic outcome, and information on factors influencing the device ID is helpful in achieving this goal. As outlined in this review, the operator can control many procedure-related factors to facilitate the implantation process. This review highlights new insights and new data regarding different aspects influencing the final ID, which can potentially positively impact the outcome when used in conjunction with individual patient-tailored implantation strategies.