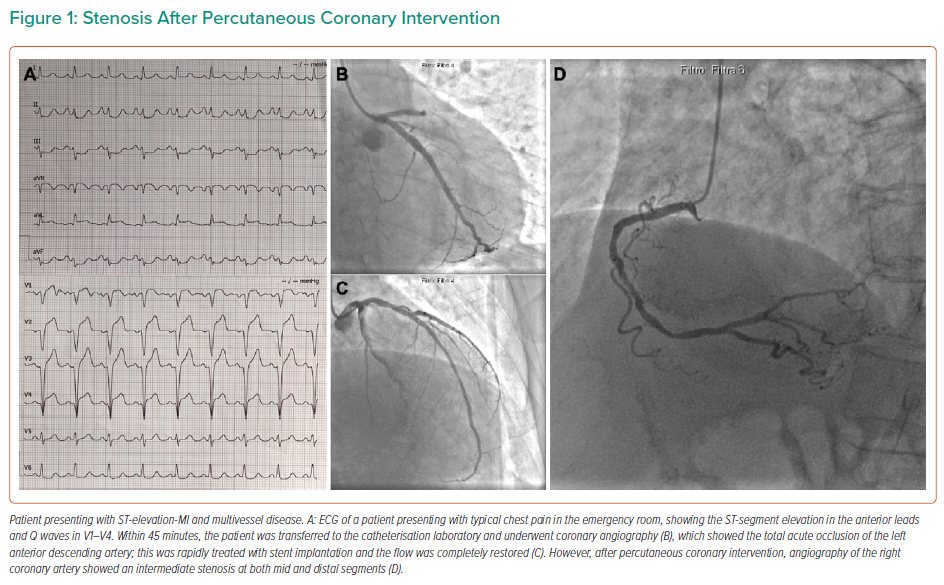
Multivessel coronary artery disease (MVD) occurs in about half of patients presenting with ST-elevation MI (STEMI) and undergoing primary percutaneous coronary intervention (PCI), and this affects both in-hospital and long-term clinical outcomes.1,2
While treating the infarct-related artery (IRA) is obviously recommended, current evidence supports revascularisation of residual significant non-culprit coronary artery lesions (NCLs). However, the ideal tool for the assessment of such residual stenoses, as well as the best time for their revascularisation remain controversial, so incomplete coronary revascularisation after primary PCI continues.
In this review, we discuss the current evidence about the benefits of complete revascularisation (CR) in patients presenting with STEMI and MVD, and examine tools for the assessment of NCLs.
Complete Coronary Revascularisation versus Infarct-related Artery-only PCI
Recent findings suggest that CR in patients presenting with STEMI and MVD is associated with a better clinical outcome than primary PCI of the IRA only, regardless of whether CR was carried out during the index procedure, the index hospitalisation or during a later readmission (Table 1).3–7
In a small randomised clinical study of 214 patients presenting with STEMI, Politi et al. divided subjects into three groups: PCI of the IRA only (culprit-only revascularisation, n=84); staged revascularisation of the NCLs (n=65); and simultaneous treatment of NCLs (CR, n=65). Residual stenoses were considered angiographically significant if the diameter of the stenosis (DS) was visually estimated to be >70%.8 At the follow-up (2.5 ± 1.4 years), 70 (32.7%) patients had experienced at least one major cardiac adverse event (MACE): 42 (50.0%) in the culprit-only revascularisation group; 13 (20.0%) in the staged revascularisation group; and 15 (23.1%) in the CR group (p<0.001).8 The incidence of in-hospital death, repeat revascularisation and rehospitalisation was significantly higher in the culprit-only revascularisation group, whereas there was no significant difference in terms of reinfarction between the three groups, suggesting that culprit vessel-only PCI is associated with a higher rate of clinical events than multivessel treatment.8
In the PRAMI trial, 465 patients presenting with STEMI undergoing primary PCI of the IRA were randomised to either preventive PCI (234 patients) or no preventive PCI (231 patients).3 Patients were eligible after primary PCI if the IRA had been successfully treated and there was a residual stenosis visually estimated to be ≥50% in at least one coronary artery other than the IRA. Recruitment was stopped early after a recommendation from the data and safety monitoring committee based on a highly significant between-group difference (p<0.001) in terms of incidence of the primary endpoint favouring preventive PCI. At a median of 23 months follow-up, the primary outcome, a composite of cardiac death, non-fatal MI or recurrent angina, occurred in 21 patients (9%) in the preventive PCI group and 53 (23%) in the group receiving PCI of the IRA only.3
In the DANAMI-3-PRIMULTI trial, performed at two Danish centres, 627 STEMI patients were randomised to no further invasive treatment after primary PCI of the IRA (n=313) or fractional flow reserve (FFR)-guided complete revascularisation (n=314).5 In this study, coronary lesions with a visually estimated DS >90% were considered angiographically significant, while stenoses of 50–90% underwent FFR assessment. At a median follow-up of 27 months, the primary endpoint (a composite of all-cause mortality, reinfarction or ischaemia-driven revascularisation of NCLs) was met in 68 (22%) patients undergoing PCI of the culprit lesion only and in 40 (13%) patients assigned to complete coronary revascularisation (HR 0.56; 95% CI [0.38–0.83]; p=0.004).5 Of note, CR resulted in a 69% risk reduction for repeat revascularisations. No significant difference in cardiac death between the two groups was observed, but the need for both urgent and non-urgent PCI of NCLs was significantly lower in the complete revascularisation group.5
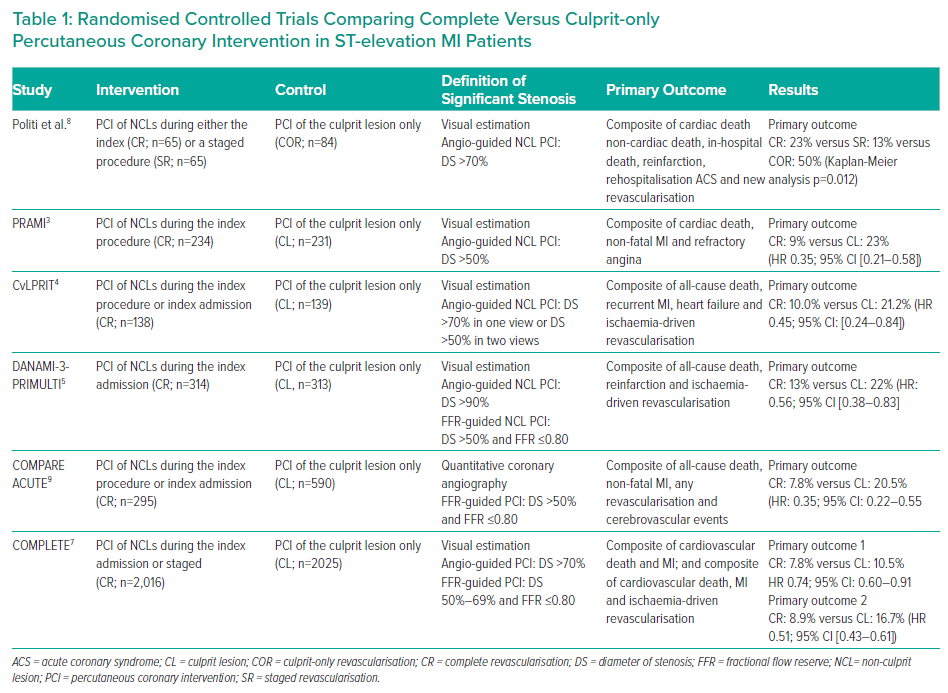
In the CvLPRIT trial, 296 patients in seven UK centres were randomised to either in-hospital complete revascularisation (n=150) or IRA-only revascularisation (n=146), with the relevance of residual coronary stenoses assessed by angiographic evaluation.4 CR was performed either at the time of primary PCI or before hospital discharge.4 Residual stenoses were considered angiographically significant if the DS was estimated visually to be >70% (in one view) or >50% (in two views). At 1-year follow up, MACE was significantly lower in the CR group (10.0%) than in the IRA-only group (21.2%; HR: 0.45; 95% CI [0.24–0.84]; p=0.009).4 Cardiovascular mortality was also numerically lower in the CR group. Moreover, a trend towards a lower MACE rate was also found in patients undergoing CR during the index procedure than in those having a staged procedure.4
In the COMPARE-ACUTE trial, 885 patients presenting with STEMI and MVD who underwent primary PCI were assigned, at a 1:2 ratio, to FFR-guided CR (n=295) or to medical therapy (n=590).9 Residual stenoses were considered angiographically suitable for FFR assessment if their DS was >50% by a visual estimation or quantitative angiography. The primary outcome occurred in 23 patients in the CR group and in 121 patients in the IRA-only PCI (HR 0.35; 95% CI [0.22–0.55]; p<0.001).9 This difference was driven mainly by a significant reduction in risk of needing new revascularisations.
More recently, the COMPLETE trial showed that, among patients with STEMI and MVD, CR was superior to IRA-only PCI in reducing the risk of cardiovascular hard endpoints at a median follow-up of 3 years.7 Residual stenoses were considered significant if, by visual estimation, DS was >70% or FFR was ≤0.80 in cases of stenoses of 50–69%. Furthermore, in this study, the investigators had to specify if they intended to perform PCI of non-culprit stenosis, either during the index procedure or 45 days later, should the patient be allocated to complete revascularisation; this allowed the investigators to evaluate whether the treatment effect of complete revascularisation versus culprit-lesion only PCI differed depending on the intended timing of non-culprit PCI.
In patients who were intended to undergo PCI during the index hospitalisation, the incidence of the two primary outcomes (cardiovascular death or MI; or cardiovascular death, MI or ischaemia-driven revascularisation) were 2.7% and 3.0% per year, respectively, in patients randomised to complete revascularisation, compared to 3.5% and 6.6% per year in those undergoing culprit-lesion-only PCI.7 The p-values for interaction for the effect of timing on the two outcomes were p=0.62 and p=0.27, respectively, suggesting a benefit of CR regardless of whether non-culprit PCI was performed during the index hospitalisation or within 45 days after randomisation. The authors explain this benefit was the result of well-treated patients with evidence-based therapies, including dual antiplatelet therapy with aspirin and a P2Y12 inhibitor, with the latter being either ticagrelor or prasugrel in the vast majority of the patients. This might have protected against early thrombotic events related to non-culprit stenosis before staged PCI.
Finally, three meta-analyses, mainly based on the cited trials, found CR was associated with a lower risk of repeat revascularisation, non-fatal MI and cardiovascular mortality compared to culprit-only PCI.10–12
Accordingly, both the European and the American guidelines now recommend PCI of NCLs should be considered in patients with STEMI and MVD before hospital discharge, either at the time of the primary PCI or in a staged procedure.13–15 However, the optimal strategy for the assessment of NCLs as well as the best timing for obtaining complete revascularisation are still matter of discussion.
Invasive Evaluation of Non-culprit Lesions
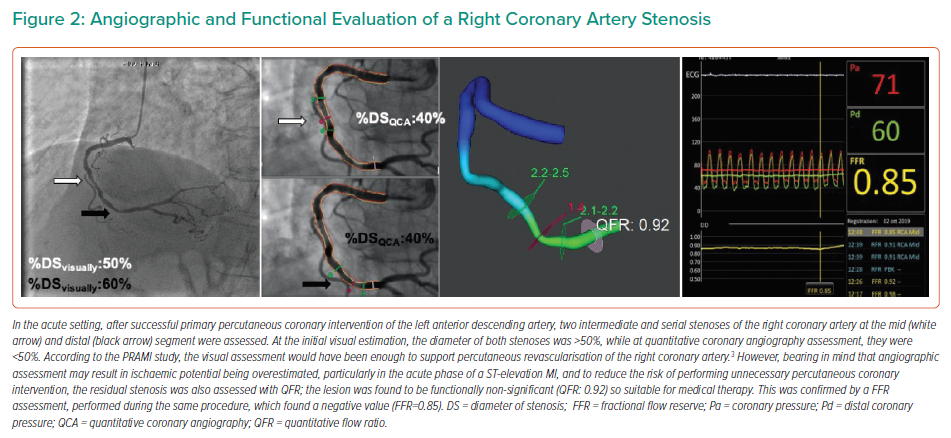
In patients presenting with STEMI when NCLs are present (Figure 1), different clinical strategies can be considered. After the treatment of the IRA, one option is initial optimal medical therapy, with further revascularisation driven by symptom recurrence. Alternatively, the decision about the need for NCL revascularisation may be based on either angiographic or functional lesion assessment (Figure 2). In all cases, NCLs should be assessed during the index procedure or within a few days of the index hospitalisation, as current guidelines suggest.13–15
Pitfalls of Angiographic Assessment of Non-culprit Lesions
During the acute phase, severity of NCLs may be overestimated by approximately 10%, especially if visual evaluation is performed by operators with low FFR experience.16–18 Consequently, angiography-guided NCL PCI in the acute setting might lead to functionally non-significant stenoses being treated.
While the PRAMI and the CvLPRIT trials were based on angiographic definition of the significant residual stenosis (DS >50%), and the DANAMI-3-PRIMULTI and COMPARE ACUTE trials were based on haemodynamic assessment of residual coronary artery stenosis at the FFR evaluation (Table 1).3–5,9 However, in the COMPLETE trial, while residual stenoses with a DS >70% were considered angiographically significant, those ones with a DS of 50–70% (<1%) were functionally assessed with the FFR.
Of note, when angiographic NCL evaluation was supplemented with FFR, 31% of patients randomised to CR in the DANAMI-3-PRIMULTI and 44% in the COMPARE-ACUTE trial did not need further PCI.5,9 This demonstrates angiographic evaluation of NCLs overestimates their ischaemic potential. It also showed that if the functional assessment of NCLs is postponed until a few days after the acute setting, the risk of performing a useless invasive procedure because a negative FFR value is found ranges between 30% and 50% of cases. Furthermore, functionally non-significant NCLs may have been treated in the angiography-guided PRAMI, CvLPRIT and COMPLETE trials, suggesting that physiology would have led to the same benefit of CR although with less PCI.3,4,7
This is in line with the evidence that in patients with stable angina undergoing FFR-guided PCI, the residual angiographic SYNTAX score (rSS) was not predictive of adverse clinical outcome; this suggests that the functional significance of a coronary lesion is definitively the most important feature for predicting future adverse cardiac events, more so than angiographic severity, and supporting the concept of functionally complete coronary revascularisation.19
Because of the frequent mismatch between the angiographic and haemodynamic severities of coronary stenoses, invasive functional evaluation, even in the setting of ACS, should be considered for all residual lesions with a DS of 50–90%, as recommended by the European guidelines for the diagnosis and management of chronic coronary syndromes.20,21
Functional Assessment of Non-culprit Lesions Using Pressure Wire
FFR is the gold standard for the invasive assessment of the ischaemic potential of a coronary artery stenosis. It is defined as the ratio between the mean distal coronary pressure and the mean aortic pressure during maximal hyperaemia and it has been shown to be useful in several clinical and anatomic contexts.22–26 Recently, a number of non-hyperaemic indices have been introduced and, as these are favoured because of a high correlation with the FFR but have the disadvantage of the need to induce maximal hyperaemia, they are more often used in the catheterisation laboratory.27,28
In the context of STEMI, functional assessment of the culprit vessel is not indicated because the dynamic changes of microcirculatory dysfunction are assessed by the index of microcirculatory resistance, leading to possible underestimation of the ischaemic potential of residual stenosis.29,30 However, a large amount of data exists for the use of such tools for the assessment of NCLs, even though most of the current evidence supporting their use has been derived from patients presenting with chronic coronary syndrome.
However, concerns have arisen in the past about the use of FFR for the assessment of NCLs in the context of ACS, particularly in the very acute phase. In fact, it has been thought that transient coronary microcirculation dysfunction might be detected even in myocardial territories supplied by non-culprit arteries, probably due to increased neurohumoral activation and/or extravascular compression secondary to myocardial oedema.31–35 In this clinical setting, temporary impairment of the microcirculation subtended by an equivocal stenosis would lead to the ischaemic potential of the coronary lesion being underestimated.
In another study, van der Hoeven et al. showed that FFR values measured for the assessment of NCLs during the index procedure were significantly higher than those measured at 30 days’ follow-up, with a mean decrease of 0.03 units; this was particularly so in patients with larger infarcts, suggesting that the ischaemic potential of NCLs might be underestimated if FFR is used during the acute setting.36
However, Ntalianis et al. found there was no significant difference between FFR values measured for the assessment of NCLs during the index procedure and after one month in patients presenting with ACS.35 Similarly, in an elegant Yorkshire pig model, Lee et al. showed that local microvascular damage induced by selective intracoronary injection of microspheres increased both the FFR and the index of microcirculatory resistance, while both remained stable in the other vessels.37 In the Wave study, Musto and colleagues showed both the FFR and instantaneous wave-free ratio (iFR) values of NCLs did not significantly change between the index and staged procedure.38 Finally, Mejía-Rentería et al. also support the use of FFR to assess NCLs during the subacute phase of MI; they also observed that, unlike the hyperaemic flow, which is preserved during the subacute phase of MI, the resting coronary flow is increased, which may have implications for the use of non-hyperaemic indices for the assessment of NCLs.39 In the iSTEMI study, the iFR value of NCLs increased by a median of 0.01 from the index procedure when re-evaluation was performed within 16 days but rose by a median of 0.03 when re-evaluation was performed >16 days after primary PCI of the culprit stenosis.40
Taken together, these studies suggest that deferring NCL revascularisation based on both the FFR and the iFR is possible even during the acute phase of a STEMI. It should be borne in mind that the ischaemic potential of residual stenosis might be overestimated when assessed by non-hyperaemic indexes.
While the clinical relevance of acute iFR-guided PCI of NCLs is being evaluated in the iMODERN trial (NCT03298659), the benefits of deferring PCI for NCLs based on the FFR measurement have already been demonstrated in previously discussed large, randomised trials.5–9 In addition, in a recent sub-analysis of three trials (FAME, FAMOUS-NSTEMI and DANAMI-3-PRIMULTI), a total of 547 patients presenting with ACS (271 patients with non-ST-elevation MI and 276 patients with STEMI) underwent FFR-guided functionally complete coronary revascularisation.41 Patients with and without MACE at 2-year follow-up had a similar rSS after PCI (rSS 7.2 ± 5.5 versus 6.6 ± 5.9; p=0.23), and a Kaplan-Meier curve analysis showed a similar incidence of MACE regardless of rSS subgroup (p=0.54).41 Therefore, even in the context of ACS, the extent of residual angiographically significant disease is not a predictor of clinical events.
Particular attention should be paid to this when with caring for elderly patients presenting with STEMI and MVD, since the benefits of complete revascularisation, whether guided by the FFR or not, remain a matter of discussion.
A recent sub-analysis from the DANAMI-3-PRIMULTI trial showed no significant differences in the incidence of the primary endpoint in elderly patients (aged ≥75 years) randomised to culprit-only or FFR-guided complete revascularisation.42 However, in the main study, fewer than 20% of patients were aged ≥75 years, so the question of whether FFR-guided complete revascularisation is effective also in this group has still to be answered. In the FIRE trial, the investigators aim to provide robust evidence on whether a specific revascularisation strategy should be applied to elderly patients presenting with MI and MVD to improve their clinical outcomes.43
Wire-free Functional Evaluation
Quantitative Flow Ratio
Quantitative flow ratio (QFR) is a novel angiography-based tool for the functional assessment of coronary artery stenoses. It has been shown to correlate with FFR, and was validated in the FAVOR and FAVOR II studies.44,45 It is based on 3D vessel reconstructions derived from angiography and the contrast flow velocity estimated by the frame count.
Two small studies assessed the predictive value of the QFR compared with FFR to identify functionally significant NCLs. In a sub-analysis of the iSTEMI study, acute QFR showed a good diagnostic performance with both staged QFR and staged FFR as references, and a moderate diagnostic performance with staged iFR as the reference.46
Spitaleri et al. published a proof-of-concept study about the application of QFR for the assessment of NCLs in patients presenting with STEMI.47 They showed an agreement between the QFR values assessed during the index (acute) and staged (3–4 days later) procedures. In addition, in a different cohort of patients, the authors found a good correlation between the FFR and QFR values of NCLs. Finally, in another cohort of patients, they showed that those with a NCL presenting with a QFR value ≤0.80 were at a higher risk of patient-oriented cardiac events (HR 2.3; 95% CI [1.2–4.5]; p=0.01).
Similarly, in the QIMERA study, QFR reassessment during a staged procedure reduced the number of significant NCLs as assessed by angiography and showed good agreement with FFR and, all patients with a QFR-negative (QFR values ≥0.82) stenosis during the index procedure remained nonsignificant at a staged assessment.48 However, 3D-QFR requires training, might be time consuming and, at least in part, is operator-dependent, which means that the assessment may be different depending on the operator who analyses the vessel (because of his experience and skills).
Other Angiographic Scores
Recently, two angiographic tools, the Angiography-DeriveD hEmoDynamic index (ADDED index) and the DILEMMA score, have been shown to predict the FFR value.
The ADDED index is defined as the ratio between the Duke Jeopardy score, which accounts for the myocardium subtended by the coronary artery stenosis and the minimal lumen diameter acquired by quantitative coronary analysis.49 With a cut-off value of 2.23, the ADDED index shows good diagnostic performance for predicting a positive or negative FFR value, with overall accuracy, sensitivity and specificity of 86%, 94% and 82%, respectively.49 In patients presenting with STEMI and MVD, it has recently been shown that deferring treatment of residual stenosis on the basis of the ADDED index, rather than the visually estimated DS, is associated with a favourable clinical outcome.50
Similarly, the DILEMMA score takes into account the minimal lumen diameter, the lesion length and BARI (Bypass Angioplasty Revascularisation Investigation) Myocardial Jeopardy Index, and it was found to have a good correlation with FFR and a discrete accuracy in predicting significant FFR values.51
Such scores could help operators to discriminate functionally significant residual coronary stenosis in patients presenting with STEMI and MVD or be used to identify patients who can be safely discharged home, avoiding useless PCI or adjunctive invasive or non-invasive procedures to assess the ischaemic potential of non-culprit coronary stenosis. However, such roles have not been investigated yet.
Intracoronary Imaging to Guide Revascularisation of Non-culprit Lesions
Intracoronary imaging has also been proposed as tool for detecting significant stenosis, namely those with the features of high-risk vulnerable plaques and significantly associated with the occurrence of acute coronary syndromes (Table 2).52
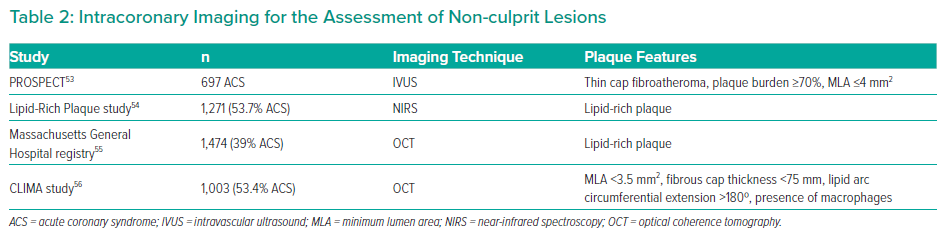
Intravascular ultrasound (IVUS) is a well-established imaging technique used in the assessment of coronary plaque features. Beyond the possibility to estimate both vessel size and plaque burden, virtual histology IVUS allows operators to identify thin-cap fibroatheroma (TCFA), fibrotic plaque and fibrocalcific plaque. In the PROSPECT study, the presence of a plaque burden of ≥70%, a TCFA and a minimal lumen area ≤4 mm2 have been suggested as independent predictors of MACE related to NCLs in patients presenting with ACS.53
Near-infrared spectroscopy has been also proposed to detect lipid-rich plaques. In the Lipid-Rich Plaque study, the risk of non-culprit-related MACE at two years increased of 18% for each 100-unit increase in maximum lipid core burden index.54
Optical coherence tomography (OCT) is a light-based imaging technique and is currently the most reliable imaging modality for TCFA detection. In the Massachusetts General Hospital OCT registry, which includes more than 1,400 patients (40% ACS), the presence of a non-culprit, lipid-rich plaque was independently associated with an increased risk of MACE related to non-culprit stenosis at 2 years.55 In the CLIMA study of >500 patients with ACS, a minimal lumen area of <3.5mm2, a fibrous cap thickness <75 mm, a lipid arc circumferential extension >180° and OCT-defined macrophage infiltration were all associated with an increased risk of MACE.56
In a OCT sub-study of the COMPLETE trial, among STEMI patients with MVD, it was found that half of patients had non-culprit lesions containing TCFA, which was more often detected in angiographic significant stenosis (DS >70% at visual estimation) than in non-obstructive lesions.57 However, it should be underlined that both OCT and IVUS-derived indexes of plaque vulnerability have a high negative predictive value for MACE but only a low positive predictive value, which limit their clinical applicability.53,56
Non-invasive Assessment of Non-culprit Lesions
Before FFR was introduced, functional evaluation of intermediate coronary artery disease relied on non-invasive tests to identify the presence of stress-inducible myocardial ischaemia. Among them, exercise ECG can be considered as their forefather. Exercise ECG can be carried out 3–5 days after an uncomplicated ACS according to the American College of Cardiology and the American Heart Association guidelines (even though it should be submaximal).58
The evidence of a stress test at an acceptable cardiovascular workload (five or more metabolic equivalents) without any ECG changes, angina, hypotension, significant ST-segment depression or frequent ventricular premature contractions may show a post-MI patient is at a low risk of recurrent cardiac events; however, there are consistent limitations to using exercise ECG to assess the functional relevance of residual coronary disease. First, exercise ECG does not have spatial resolution to correctly identify myocardial ischaemia, especially in patients with MVD; in addition, changes to the ECG at rest after MI might decrease the sensibility and the predictive value of the test.
Dobutamine stress echocardiography has been proven to be safe when performed 5 days after MI.59 Previous studies have shown that stress echocardiography might be an efficient tool to detect the presence of myocardial ischaemia, including in myocardial territories not supplied by the culprit artery in patients with MVD.60
Coronary flow velocity reserve (CFVR) by transthoracic Doppler echocardiographic imaging might be also useful in the assessment of non-culprit coronary artery stenosis. Tesic et al. enrolled 230 patients with residual intermediate (50%–70%) stenosis of the non-infarct-related arteries, in whom CFVR was performed within 7 days of primary PCI. The authors found that deferring patients with intermediate residual stenosis with a CFVR >2 was safe and associated with excellent long-term clinical outcomes. However, this tool is particularly affected by some limitations due to the acoustic window of the patients and the feasibility of the technique to assess flow in different coronary arteries. The evaluation of CFVR is more feasible in the left anterior descending artery and right coronary artery than the left circumflex artery.61
Similarly, quantitative myocardial single photon emission CT (SPECT) has been used largely to detect residual myocardial ischaemia after acute MI.62 Stress echocardiography and myocardial SPECT are equally accurate for detecting MVD early after acute MI.60
However, unlike with the FFR-guided strategy, there are no studies evaluating the prognostic impact of a non-invasive, imaging-based strategy to guide myocardial revascularisation of residual non-culprit coronary artery disease in patients with STEMI.63 The same applies to perfusion cardiac MRI.
FFR derived from computed coronary angiography (FFR-CT) deserves the final mention in this setting. Computation of the FFR from standard acquired coronary CT angiography datasets has recently been developed. The diagnostic performance of FFR-CT in identifying functional significant stenosis using the FFR as the standard of reference is high and superior to anatomical interpretation in patients with stable angina; in addition, its application for the evaluation of NCLs in patients presenting with STEMI has recently been evaluated in a prospective, single centre study where patients undergoing primary PCI with at least one equivocal stenosis in a non-culprit vessel were subjected to coronary CTA after 1 month.64 Using computational fluid dynamics principles, coronary blood flow and pressures were computed under simulated hyperaemic conditions; lesion-specific ischaemia was defined as FFR-CT <0.80 as in previous studies. However, in this study, the overall diagnostic performance of FFR-CT for staged detection of functional significant NCLs appeared to be modest.64
Cardiac MRI might also be considered to evaluate patients with suspected obstructive coronary artery disease. Cardiac MRI does not expose patients to ionising radiation and allows high-resolution imaging to be obtained. It is also possible to quantify myocardial blood flow in both relative and absolute terms.65,66 In a sub-study of the REDUCE-MVI trial, Everaars et al. found a moderate agreement between CMR and FFR for the assessment of non-culprit stenosis in patients presenting with STEMI and MVD. However, the sample size was limited and mainly underpowered for this purpose, so randomised trials would be useful for supporting this tool for the assessment of non-culprit stenosis in the setting of ACS.67,68
Conclusion
The correct management of residual coronary artery disease in patients with STEMI and MVD undergoing primary PCI remains a concern. Issues remain regarding the correct timing and guiding criteria for interventions. The introduction of FFR together with the concept of functionally complete coronary revascularisation is surely a critical innovation. However, functional measures, such as the FFR and/or its surrogates should not completely supplant clinical judgement.
Lesion vulnerability, patient comorbidities, size of ischaemic territory, ability to comply with dual antiplatelet therapy and risk of contrast-induced kidney injury are only some of the issues that should be considered when pursuing complete revascularisation.
Larger studies, such as FULL REVASC (NCT02862119) and FRAME-AMI (NCT02715518), will add further knowledge to this complex and interesting field.