Case Presentation
A 47-year-old woman with recurrent angina at rest and on exertion was referred to the Department of Cardiology, Radboud University Medical Center for a second opinion on coronary vascular dysfunction.
Her medical history included coronary artery disease and percutaneous coronary intervention (PCI) of the first diagonal branch (D1) 2 years before presentation. Repeat invasive coronary angiography had been performed in the referring centre and showed non-significant coronary artery disease in the left anterior descending artery (LAD) segment 7 just distal to the bifurcation with D1.
In the absence of significant obstructive coronary artery disease, the patient was tentatively diagnosed with coronary microvascular dysfunction and treatment was started with diltiazem and nitrates. Despite the treatment, the patient had recurrent episodes of angina. We decided to perform invasive coronary angiography, including coronary artery spasm provocation testing and assessment of microvascular function.
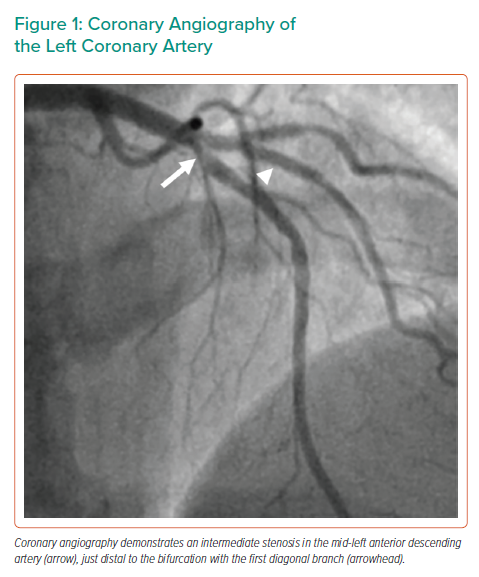
The invasive coronary angiography was performed using right radial access. The right coronary artery showed mild atherosclerosis without significant stenosis. The left main coronary artery showed no significant stenosis. In LAD segment 7 just distal to the bifurcation with D1, there was an intermediate stenosis (Figure 1 and Supplementary Material Video 1). There was no significant stenosis in the circumflex artery.
Acetylcholine testing, using incremental acetylcholine bolus administration (2 μg, 20 μg and 100 μg), revealed diffuse epicardial coronary artery spasm, most profound just distal to the stent in D1 and in the mid-LAD, which was accompanied by anterolateral ST-depressions. Intracoronary nitroglycerine administration resolved the coronary artery spasm.
After coronary artery spasm provocation testing, a PressureWire X (Abbott Vascular) was introduced into the LAD for physiological assessment of the stenosis in the mid-LAD as well as the microvasculature.
The patient had a normal coronary flow reserve (4.0; normal >2.5) and normal microvascular resistance (19, corrected 17; normal <25), ruling out coronary microvascular dysfunction. Physiological assessment of the lesion in the mid-LAD revealed haemodynamic significance with a fractional flow reserve of 0.75 (normal >0.80) and a resting full cycle ratio of 0.84 (normal >0.89).
In the absence of dual anti-platelet therapy, a staged procedure was scheduled. During this procedure, optical coherence tomography (OCT) of the LAD was performed using a Dragonfly Optis Imaging Catheter (Abbott Vascular) to assess the underlying mechanism of stenosis.
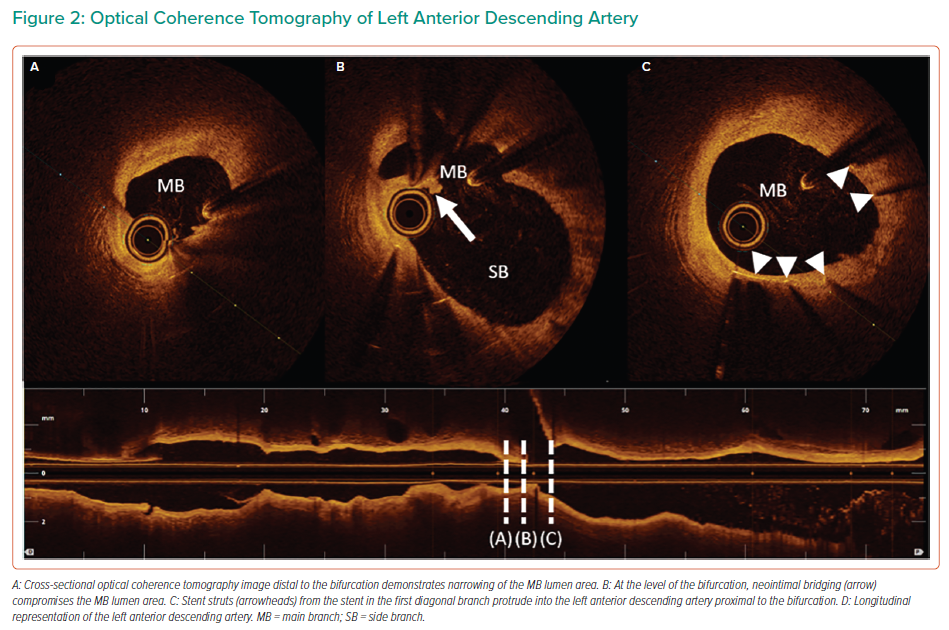
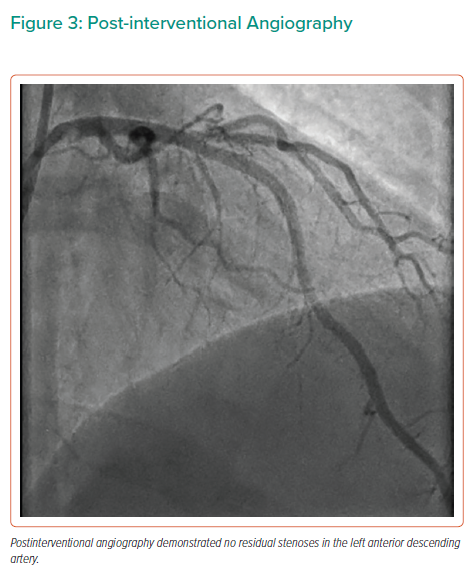
OCT demonstrated a significant reduction in the LAD lumen area just distal to the D1 bifurcation, which was caused by protrusion of the previously implanted stent into the LAD, resulting in neointimal bridging over the protruding stent struts (Figure 2 and Supplementary Material Video 2). Accelerated downstream atherosclerosis was also seen. The minimal, proximal reference and distal reference lumen area measured 2.73 mm2, 10.02 mm2 and 7.09 mm2, respectively. Provisional PCI of the LAD was performed using a cross-over stenting strategy with a single 3.0–38 mm XIENCE Sierra (Abbott Vascular) drug-eluting stent, thereby performing a minicrush on the D1 stent. Subsequently, a proximal optimisation technique just proximal to the carina using a 3.5 mm non-compliant balloon was performed without side branch recrossing or kissing balloon inflations, thereby achieving good angiographic success (Figure 3 and Supplementary Material Video 3). During follow-up, the patient remained free of angina with continuous nitrates and diltiazem prescription.
Discussion
Full physiological assessment, including coronary artery spasm provocation testing, identified two mechanisms of angina in a patient who previously underwent PCI. First, significant diffuse epicardial spasm was diagnosed, and may have caused the anginal symptoms at rest. Second, a significant stenosis of the mid-LAD was detected using physiological assessment, which explains the exertional angina. OCT revealed that stent protrusion from the D1 into the LAD, which resulted in neointimal bridging over the protruding stent struts and accelerated downstream atherosclerosis, was the underlying mechanism of stenosis.
Stent Protrusion
The main concern of stent protrusion is the risk of acute stent thrombosis with potential life-threatening sequelae.1 Two mechanisms explain the relationship between stent protrusion and stent thrombosis. First, protruding stent struts alter coronary blood flow, resulting in increased shear stress at the stent boundaries and downstream, as well as impaired stent strut coverage.2 Shear stress is associated with platelet activation and subsequent thrombus formation, especially during prolonged exposure.3 Second, incomplete strut coverage, by itself, is associated with the occurrence of late stent thrombosis.4 The effect size of these two mechanisms increases with larger distances from the stent struts to the vessel wall.2
Stent protrusion may also result in gradual stenosis, as demonstrated in our patient. First, although delayed, stent strut coverage will occur over time and may result in neointimal bridging.5,6 Bridging may result in flow disturbances by creating a neocarina. Moreover, neointimal filling of the newly formed compartments further decreases the flow area. This effect is especially evident in the presence of stent strut links in the protruding segment.6–9 Second, regions of low shear stress at recirculation zones, which are predominantly found just distal to a stent strut, promote neointimal formation and neoatherosclerosis.10,11
OCT for the Evaluation of Stent Failure
Although angiography provides a low-resolution luminogram, intravascular imaging by OCT provides high-resolution cross-sectional images, thereby enabling evaluation of stent failure (both in-stent restenosis and thrombosis).
In-stent restenosis can be caused by neointimal hyperplasia or neoatherosclerosis, and is strongly associated with stent underexpansion.12–14 Identification of the aspect of in-stent restenosis has prognostic implications, considering that heterogeneous neointimal hyperplasia is assocciated with less-favourable outcomes compared with a homogeneous or layered neointima.15 Similarly, neoatherosclerosis is independently associated with target lesion revascularisation.16
OCT is also valuable for the evaluation of stent thrombosis. Observational in vivo studies and registries have identified independent associations between stent thrombosis on the one hand, and malapposition, underexpansion or stent edge dissections on the other.17–19
In the PESTO registry, OCT identified an underlying morphological abnormality in 97% of stent thromboses after treatment of the culprit lesion.20 Although the percentage of patients in whom a direct cause was considered to be identified increased after OCT, local operators considered a causal mechanism unidentified in 13% of patients.20
However, it has to be acknowledged that the presence of a thrombus hampers visualisation of the vascular wall and stent struts. Therefore, immediate evaluation may not be feasible in as many cases in daily clinical practice. Yet, if visible, information on the mechanism of stent failure can be used to guide treatment strategies, including high-pressure post-dilation or additional stenting. As such, contemporary guidelines endorse the use of intravascular imaging for the evaluation of restenosis.21
Acetylcholine Testing
In the present case, acetylcholine testing was performed prior to functional assessment of the LAD stenosis. It has been argued that the presence of intermediate stenoses provokes coronary spasms and that spasms in such circumstance do not necessarily represent endothelial dysfunction. Indeed, epicardial spasms are frequently found at sites with significant atherosclerotic disease.22 However, in the present case, coronary artery spasms were found at other segments that were free from atherosclerosis. In addition, contemporary expert opinion papers highlight the lack of evidence in the optimal sequence of invasive testing.23
Conclusion
We report a case in which full functional testing identified two mechanism of angina in a patient who had previously undergone percutaneous revascularisation, one of which was a haemodynamically significant stenosis distal to the LAD–D1 bifurcation. OCT was valuable for the identification of the underlying mechanism of obstruction with main branch compression by a protruding stent, accompanied by neointimal bridging and induced accelerated downstream atherosclerosis, which further impacted the remaining lumen area. As such, ostial side branch stenting with stent protrusion may initially provide a happy result, but the downsides could also appear at a later time.
Clinical Perspective
- Optical coherence tomography is valuable for detecting stent protrusion as the underlying mechanism for obstructive coronary artery disease.
- Neointimal bridging may compromise the main branch in case of stent protrusion.
- After side branch stenting, stent protrusion into the main branch is not to be neglected.